Like hookworms, Strongyloides larvae enter the body through exposed skin (for example, when you walk barefoot). Infection is often associated with agricultural work. People infected with HTLV-1 are more likely to acquire Strongyloides and are more prone to severe strongyloidiasis. In contrast, HIV/AIDS has not been shown to increase the risk of acquiring strongyloidiasis or to worsen the clinical course.
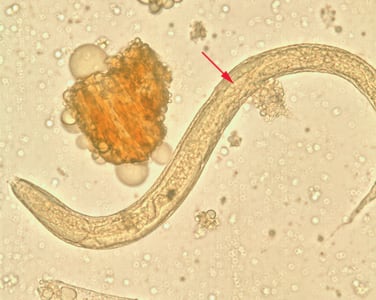
Pathogenesis: Strongyloides stercoralis is the most commonly implicated species. Infective filariform larvae penetrate the skin and migrate through the body to reach the small intestine. They travel via the bloodstream and lymphatics to the lungs, are coughed up, and then swallowed. In the small intestine, the larvae molt twice and become adult female worms.
The adult females live embedded in the submucosa of the small intestine and produce eggs by parthenogenesis (parasitic males do not exist). These eggs yield rhabditiform larvae. The rhabditiform larvae have two possible fates:
In autoinfection, rhabditiform larvae in the gut become infective filariform larvae. These filariform larvae can penetrate either the intestinal mucosa or the perianal skin, enter the bloodstream, and repeat the cycle. The key clinical significance of autoinfection is that untreated infection can persist for years and may progress to hyperinfection syndrome.
Transmission of S. fuelleborni subsp. kellyi to infants through breastfeeding has been reported.
Clinical features: Infection often begins with a localized, pruritic, erythematous rash at the site of skin penetration. As larvae migrate through the lungs and up the trachea, patients may develop tracheal irritation and a dry cough. After the larvae are swallowed and reach the gastrointestinal tract, symptoms may include diarrhea, constipation, abdominal pain, and anorexia.
Chronic strongyloidiasis is generally asymptomatic, but a range of gastrointestinal and cutaneous manifestations can occur. Rarely, chronic infection is associated with other complications (e.g. arthritis, cardiac arrhythmias, chronic malabsorption, duodenal obstruction, nephrotic syndrome, recurrent asthma). Up to 75% of people with chronic strongyloidiasis have mild peripheral eosinophilia or elevated IgE levels.
Hyperinfection syndrome is often seen in patients receiving high dose corticosteroid therapy. In hyperinfection syndrome, an unusually large number of parasites are present. The larvae are limited to the GI tract and lungs in hyperinfection syndrome, whereas disseminated strongyloidiasis involves numerous other organs.
“Larva currens” presents as recurrent, serpiginous, maculopapular rashes on the buttocks, perineum, and thighs. It is caused by subcutaneous migration of filariform larvae during autoinfection.
“Swollen belly syndrome” is seen in infants with S. fuelleborni subsp. Kellyi infections. It is a fatal, systemic illness involving protein-losing enteropathy and ascites.
Laboratory diagnosis of Strongyloidiasis: S. stercoralis infections are best diagnosed by serology because larvae are infrequently passed in stool. Indirect immunofluorescence, CFT, RIA for IgE, gelatin particle agglutination test, Western blot, and ELISA can be used for diagnosis. S. stercoralis larvae can also be demonstrated in stool samples. Children with S. fuelleborni infection shed eggs (rather than larvae) in the feces, and the infection is easily diagnosed using microscopic techniques.