Plasmodium species are the causative agents of malaria. Malaria is endemic in tropical and subtropical regions worldwide, with the highest mortality in children and pregnant women. In the USA, malaria is most often seen in travelers returning from endemic areas and in recent immigrants from these regions.
Pathogenesis: Plasmodium has a complex life cycle with multiple stages and two hosts. The female Anopheles mosquito is the vector.
In the mosquito:
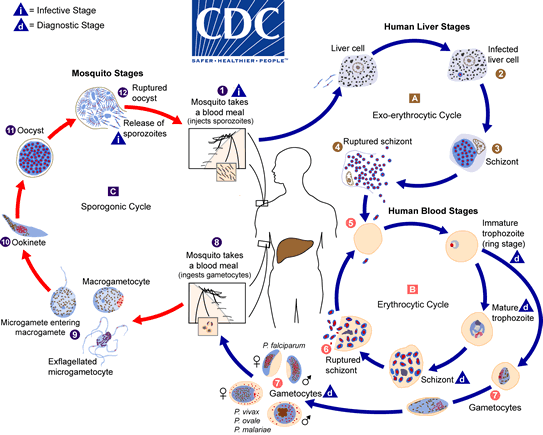
The malaria parasite life cycle involves two hosts. During a blood meal, a malaria-infected female Anopheles mosquito inoculates sporozoites into the human host The number 1. Sporozoites infect liver cells The number 2 and mature into schizonts The number 3, which rupture and release merozoites The number 4. (Of note, in P. vivax and P. ovale a dormant stage [hypnozoites] can persist in the liver and cause relapses by invading the bloodstream weeks, or even years later.) After this initial replication in the liver (exo-erythrocytic schizogony The letter A), the parasites undergo asexual multiplication in the erythrocytes (erythrocytic schizogony The letter B). Merozoites infect red blood cells The number 5. The ring stage trophozoites mature into schizonts, which rupture releasing merozoites The number 6. Some parasites differentiate into sexual erythrocytic stages (gametocytes) The number 7. Blood stage parasites are responsible for the clinical manifestations of the disease.
The gametocytes, male (microgametocytes) and female (macrogametocytes), are ingested by an Anopheles mosquito during a blood meal The number 8. The parasites’ multiplication in the mosquito is known as the sporogonic cycle The letter C. While in the mosquito’s stomach, the microgametes penetrate the macrogametes generating zygotes The number 9. The zygotes in turn become motile and elongated (ookinetes) The number 10 which invade the midgut wall of the mosquito where they develop into oocysts The number 11. The oocysts grow, rupture, and release sporozoites The number 12, which make their way to the mosquito’s salivary glands. Inoculation of the sporozoites into a new human host perpetuates the malaria life cycle.
Two classic host factors affect susceptibility:
Clinical features: Malaria often starts with flu-like symptoms, including fever, chills, sweats, headache, muscle pains, jaundice, nausea, and vomiting.
Severe malaria is primarily caused by Plasmodium falciparum and may include cerebral malaria. Findings can include confusion, coma, focal neurologic signs, renal failure, severe anemia, and respiratory difficulties. Cerebral malaria is commonly associated with hypoglycemia.
Hemolysis can lead to hemoglobinuria, which may cause renal shutdown with dark urine. This is called blackwater fever, named for the dark color of the urine.
Diagnosis of Malaria:
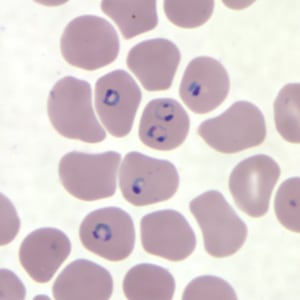
Rings of P. falciparum in a thin blood smear.
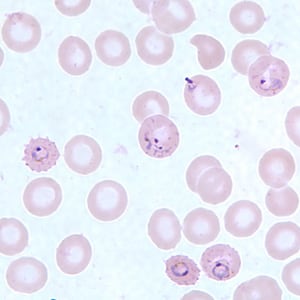
Ring-form trophozoites of P. falciparum in a thin blood smear, exhibiting Maurer’s clefts.
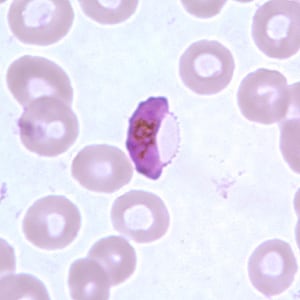
Gametocyte of P. falciparum in a thin blood smear.
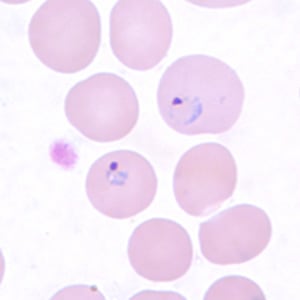
Ring-form trophozoites of P. vivax in a thin blood smear.
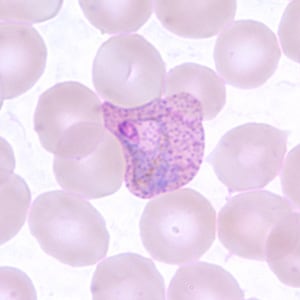
Trophozoite of P. vivax in a thin blood smear. Note the amoeboid appearance, Schüffner’s dots and enlarged infected RBCs.
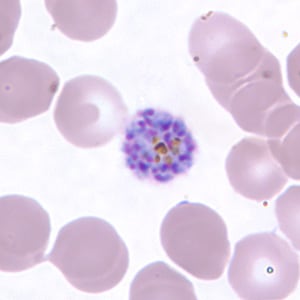
Schizont of P. vivax in a thin blood smear.
Sign up for free to take 2 quiz questions on this topic