Fundamentals
Glycolysis is used by all tissues to break down glucose, producing ATP (energy) and intermediates for other metabolic pathways. In cells with mitochondria and an adequate O2 supply, pyruvate is the end product of glycolysis. Pyruvate then undergoes oxidative decarboxylation by the pyruvate dehydrogenase enzyme to form acetyl CoA, which enters the TCA cycle to generate ATP.
In cells that lack mitochondria (e.g., RBCs) or in hypoxic conditions (e.g., AMI), aerobic metabolism can’t proceed normally. These cells rely on anaerobic glycolysis, in which pyruvate is reduced to lactate while NADH is converted back to NAD.
Steps in glycolysis:
1) First, glucose must enter the cell. It does so by two mechanisms:
a) Na Independent Facilitated Diffusion: Glucose moves down its concentration gradient (from high to low concentration). This transport is mediated by 14 tissue-specific glucose transporters, GLUT 1 to GLUT 14.
- GLUT 1: RBCs and BB barrier
- GLUT 2: liver, kidney, and beta cells of the pancreas
- GLUT 3: neurons
- GLUT 4: adipose tissue and skeletal muscle (its number is increased by insulin)
- GLUT 5: fructose transporter in testes and small intestine
b) Na Monosaccharide Cotransporter System: This is an energy-requiring process because it transports glucose against its concentration gradient (from low to high concentration). Glucose transport is coupled to Na cotransport, so it’s also called SGLUT (Sodium GLUT). SGLUT is present in the renal tubules, choroid plexus, and intestinal epithelial cells.
Once glucose is inside the cell, it must be “trapped” by phosphorylation to form glucose 6 phosphate, using either hexokinase or glucokinase.
a) Hexokinase: Present in most tissues.
- Inhibited by its product, glucose 6 phosphate
- Low Km (high affinity) and low Vmax for glucose
- This allows tissues to use glucose even when blood glucose is low
- Hexokinase serves as a glucose sensor in the hypothalamus and plays a key role in the adrenergic response to hypoglycemia
b) Glucokinase: Present in liver parenchymal cells and beta cells of the pancreas.
- In pancreatic beta cells, it serves as a glucose sensor that helps determine the threshold for insulin secretion
- In the liver, it promotes glucose phosphorylation during hyperglycemia (post-meal fed state)
- Higher Km (low affinity) and high Vmax for glucose
- Therefore, glucokinase functions mainly when hepatocyte glucose concentration is elevated (e.g., post meal)
- Activity is inhibited indirectly by fructose 6 phosphate (2nd step in glycolysis), not by glucose 6 phosphate, and is indirectly stimulated by glucose
- Mutations that decrease glucokinase activity can cause maturity onset diabetes of the young (MODY)
Role of GKRP (glucokinase regulatory protein in the liver): In the presence of fructose 6 phosphate, glucokinase is translocated to the nucleus, where it binds to GKRP and becomes inactive. When glucose levels rise, glucokinase is released from GKRP and becomes active. Fructose 1 phosphate activates glucokinase by inhibiting formation of the glucokinase-GKRP complex.
2) Glucose 6 phosphate is converted to fructose 6 phosphate by phosphoglucose isomerase.
3) Fructose 6 phosphate is converted to fructose 1,6 biphosphate by PFK 1. This is the rate-limiting step and the most important regulatory step of glycolysis.
- PFK 1 (phosphofructokinase 1) is inhibited by elevated ATP and citrate
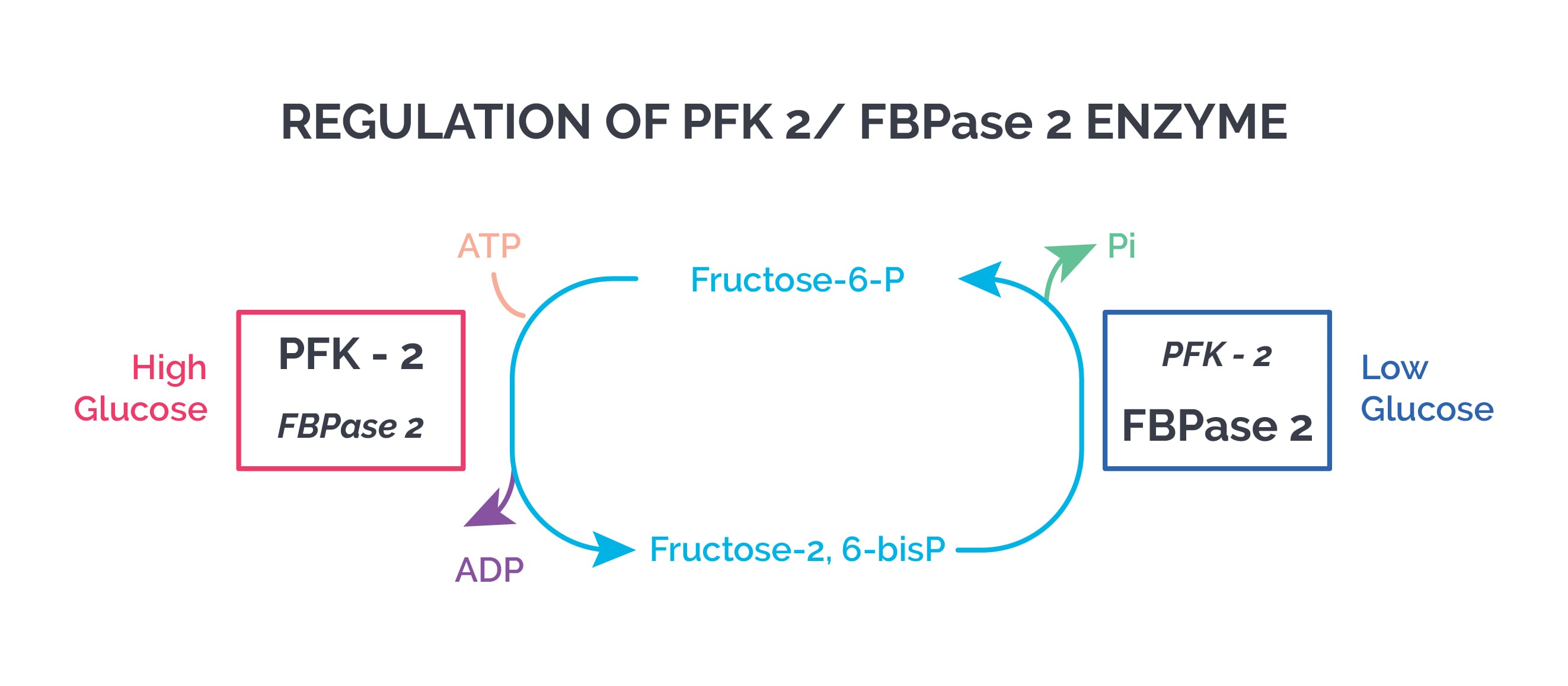
- PFK 1 is activated by AMP and fructose 2,6 biphosphate
Fructose 2,6 biphosphate is formed by PFK 2 in a reversible reaction. Fructose 2,6 biphosphate:
- Stimulates glycolysis
- Inhibits gluconeogenesis (by inhibiting fructose 1,6 bisphosphatase, a gluconeogenesis enzyme)
During the well-fed state (high insulin/post meal, low glucagon), cAMP is low and protein kinase A is inactive. This leads to dephosphorylation (activation) of PFK 2, increasing fructose 2,6 biphosphate and stimulating glycolysis.
During fasting/starvation (low insulin, high glucagon), fructose 2,6 biphosphate levels in the liver are low, which inhibits glycolysis and activates gluconeogenesis.
4) Fructose 2,6 biphosphate is cleaved by aldolase to form glyceraldehyde 3 phosphate (G3P) and DHAP (dihydroxyacetone phosphate).
5) Triose phosphate isomerase converts G3P to DHAP.
6) G3P is converted to 1,3 BPG by glyceraldehyde 3 phosphate dehydrogenase, producing NADH.
Arsenic Poisoning: Arsenic inhibits pyruvate dehydrogenase by binding to lipoic acid. Pentavalent arsenic (arsenate) prevents the formation of NADH and ATP in glycolysis by competing with inorganic phosphate as a substrate for G3P dehydrogenase. Arsenic also inhibits the ATP synthase enzyme.
2,3 BPG: In RBCs, 1,3 BPG is converted to 2,3 BPG by the enzyme BPG mutase, facilitating increased oxygen delivery to tissues.
7) 1,3 BPG is converted to 3 phosphoglycerate by phosphoglycerate kinase. ATP is produced.
8) 3 phosphoglycerate is converted to 2 phosphoglycerate by phosphoglycerate mutase.
9) 2 phosphoglycerate is converted to PEP (phosphoenolpyruvate) by enolase.
10) PEP is converted to pyruvate by pyruvate kinase. ATP is formed.
- In the liver, pyruvate kinase is activated by fructose 1,6 biphosphate (feed-forward regulation)
- When blood glucose is low, elevated glucagon increases cAMP, activating protein kinase A and causing phosphorylation and inactivation of pyruvate kinase
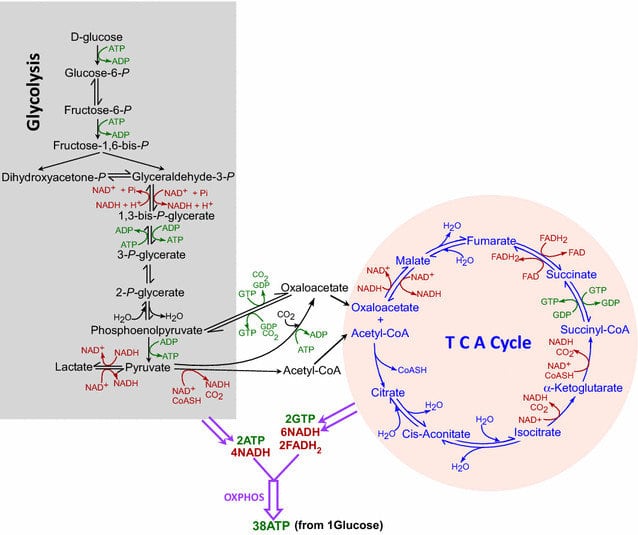
The metabolic steps of glycolysis and TCA cycle. The steps involved in glycolysis and the TCA cycle are demarcated separately. ATP/GTP utilization or synthesis is shown in green, while NAD + /NADH and FAD/FADH 2 are shown in red. Also indicated are the numbers of NADH, FADH 2, and ATP/GTP generated when one molecule of glucose is consumed following glycolysis, TCA cycle, and oxidative phosphorylation.
Pyruvate Kinase Deficiency: Mature RBCs lack mitochondria and depend entirely on glycolysis for ATP. Decreased ATP damages the RBC membrane and alters cell shape, ultimately leading to phagocytosis by splenic macrophages and hemolytic anemia. RBCs compensate by increasing 2,3 BPG. In pyruvate kinase deficiency, pyruvate kinase is mutated and may show altered kinetics such as abnormal response to the activator fructose 1,6 biphosphate; abnormal Km or Vmax; abnormal folding; reduced enzyme amounts; etc.
Pyruvate to lactate: Lactate is the end product of anaerobic glycolysis, produced by lactate dehydrogenase in the lens, cornea, renal medulla, testes, leukocytes, and RBCs.
In exercising skeletal muscle, NADH production can exceed the oxidative capacity of the respiratory chain. This increases the NADH/NAD ratio, which favors reduction of pyruvate to lactate by lactate dehydrogenase (LDH) (a bidirectional enzyme). Lactic acid in muscle may cause cramps.
When the NADH/NAD ratio is low (e.g., in heart muscle and liver), LDH favors oxidation of lactate from blood to pyruvate. In the liver, pyruvate is either converted to glucose by gluconeogenesis or oxidized in the TCA cycle.
Measurement of lactic acid levels is an indicator of “oxygen debt” and predicts mortality and morbidity in many clinical conditions.