Overexpression of the gene eotaxin-3 (chemotactic for eosinophils) and mutations in CAPN14 and TSLP have been associated with eosinophilic esophagitis. Enhanced eosinophil chemokine receptor 3 and Th2-mediated immunity are seen.
It presents with dysphagia, food impaction, odynophagia (painful swallowing), nausea, weight loss, and vomiting. Symptoms may mimic GERD.
Diagnosis is by esophagoscopy and biopsy.
Endoscopic findings include circular rings (trachealization), linear furrows, whitish plaques, papules, and strictures. Biopsy cut-off is >15 eosinophils/high power field infiltrating the esophageal mucosa and submucosa, with microabscesses and fibrotic changes. Laboratory findings include eosinophilia.
Therapy includes diet modification (avoiding allergic food ingredients), proton pump inhibitors, steroids, and esophageal dilation. Eosinophil-blocking antibodies are being investigated as drug therapy.
Risk is increased with obesity, alcohol use, tobacco smoking, excess caffeine, pregnancy, sliding hiatal hernia, systemic sclerosis, and older age.
It presents with heartburn, sour taste, retrosternal chest pain, sensation of a lump in the throat, cough, wheezing, nausea, vomiting, etc. Symptoms are exacerbated by big, fatty meals and the supine position.
Alarm symptoms include dysphagia, odynophagia, weight loss, or bleeding.
Untreated or long-standing GERD may lead to esophagitis, stricture, metaplastic changes, and cancer.
Diagnosis is mainly clinical. Ambulatory pH monitoring is considered the gold standard for diagnosing GERD. Acid reflux on pH monitoring manifests as abrupt drops in pH to a level below pH of 4. It allows dynamic monitoring of esophageal pH changes.
Upper gastrointestinal or esophageal endoscopy helps diagnose complications like esophagitis, stricture, and Barrett’s esophagus, and also enables biopsy. Endoscopy shows erythema and linear ulcers at the distal esophagus.
Histologic findings include inflammatory cells (eosinophils, neutrophils, and lymphocytes) in the epithelium, along with basal cell hyperplasia, ballooning of squamous cells, and multinucleated giant cells.
Treatment is with lifestyle modification, maintaining a healthy weight, H2 blockers, and PPIs (more effective). Refractory cases are treated surgically with Nissen fundoplication, which reinforces the lower esophageal sphincter.
Approximately 10-15% of patients with GERD develop Barrett’s esophagus. It may progress to adenocarcinoma of the lower esophagus.
Risk factors include male sex, Caucasian race, tobacco smoking, and obesity.
Screening for BE is done by endoscopy and is recommended in men with chronic GERD with at least 2 risk factors.
Criteria to diagnose Barrett’s esophagus are:
Management is with PPIs and screening for dysplasia or adenocarcinoma. Endoscopic mucosal resection or endoscopic ablation are done in the presence of dysplasia.
Risk factors include smoking, alcohol use, city dwellers, eating hot food, high-risk HPV (especially HPV-16), Plummer-Vinson syndrome, achalasia, stricture, webs, and diverticula. It is more common in African Americans.
Adenocarcinoma is seen in the lower esophagus and may develop in Barrett’s esophagus. Squamous cell carcinomas, on the other hand, are seen in the upper and middle esophagus.
Multiple, sequential gene mutations in p53, cyclin D1, HER2, cMYC, EGFR, RB, p16, etc. have been found in esophageal cancers.
Presentation is often late, which contributes to a poor prognosis. Symptoms include progressive dysphagia (difficulty swallowing solids followed over time by dysphagia to liquids), odynophagia (pain while swallowing), and weight loss.
Squamous cell carcinoma may cause esophageal perforation, fistula formation, mediastinitis, or aortic erosion.
Metastases occur early to regional lymph nodes, followed by distant metastases to the liver, lungs, and pleura. Patterns of lymph node spread include:
On endoscopy:
On histology:
It may be associated with GERD, Barrett’s esophagus, aspiration, and strictures, and it carries a risk of progression to SCC of the esophagus.
Achalasia results from lack of ganglion cells in the myenteric (Auerbach’s) plexus in the lower esophagus. Acquired cases result from Chagas disease, DM, esophageal cancers, amyloidosis, and sarcoidosis.
Clinical features include progressive dysphagia, chest pain, regurgitation (due to retention of food in the esophagus), aspiration, cough, weight loss, and aspiration pneumonia.
CxR and barium swallow show a massively dilated esophagus with a tapered, “bird’s beak” appearance.
Biopsy findings include lymphocytic infiltration in the epithelium, submucosal atrophy, absence of ganglion cells, loss of nerve fibres, collagen deposition, and muscular hypertrophy.
Esophageal manometry shows incomplete relaxation of the LES in response to swallowing, high resting LES pressure, and absent esophageal peristalsis.
Treatment includes isosorbide mononitrate, nifedipine, botulinum toxin (to relax the sphincter), esophagomyotomy (Heller myotomy or peroral endoscopic myotomy), and esophageal dilation.
Manometric schematic of achalasia demonstrating aperistaltic contractions, increased intraesophageal pressure and failure of relaxation of the lower esophageal sphincter.
It is caused by degeneration of the vagus nerve with lack of inhibitory parasympathetic innervation.
It presents with chest pain and dysphagia to solids and liquids. Hiatal hernia is often associated.
Manometry shows intermittently abnormal primary peristalsis associated with a pattern of repetitive, simultaneous, ineffective contractions of varying amplitudes.
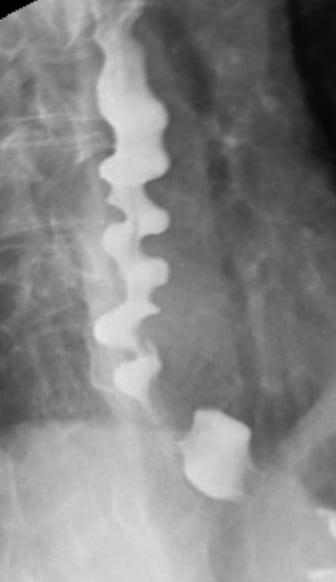
Barium swallow shows repetitive, lumen-obstructing, non-peristaltic contractions with a typical “corkscrew” or “rosary bead” appearance.
Treatment is with calcium channel blockers, hydralazine, nitroglycerine, antidepressants, and botulinum toxin.
It is more common in older females and presents with chest pain, dysphagia, and heartburn. Dysphagia occurs with both solids and liquids. It may be confused with IHD or GERD.
It often co-exists with psychiatric disturbances, IBS, and fibromyalgia.
Barium swallow is typically normal or may show abnormal motility.
Manometry shows >180 mmHg intraesophageal pressures during peristalsis, repetitive contractions, and elevated pressure in the LES, with a normally relaxing LES.
Medical treatment is with nitroglycerine, diltiazem, sildenafil, and/or antidepressants.
Sign up for free to take 4 quiz questions on this topic