Arteriosclerosis is thickening and hardening of arterial walls. It’s commonly seen in aging blood vessels due to thickening of the media and intima from deposition of collagen and elastin. This contributes to the normal age-related increase in blood pressure, especially systolic blood pressure.
Pathological types are as follows:
I) Monckeberg’s medial calcific sclerosis: Characterized by dystrophic calcification of medium and large arteries in individuals older than 50 years of age. It specifically affects the tunica media. Arteries become rigid, but there is no narrowing of the lumen.
II) Arteriolosclerosis: Affects small muscular arteries and arterioles. It may be hyaline, hyperplastic, or necrotizing.
| Type of arteriolosclerosis | Characteristic features |
| Hyaline | Seen in DM, HT; deposition of hyaline, proteinaceous material in tunica intima and media; increased endothelial permeability; narrowed vessel lumen |
| Hyperplastic | Seen in malignant hypertension, HUS, eclampsia, scleroderma; interlobular renal arteries involved; hyperplasia of smooth muscle cells of the tunica intima with “onion skin” appearance; duplication of basement membrane |
| Necrotizing | Seen in severe and malignant hypertension; fibrinoid necrosis plus hyaline sclerosis of small arteries and arterioles; neutrophilic infiltrate |
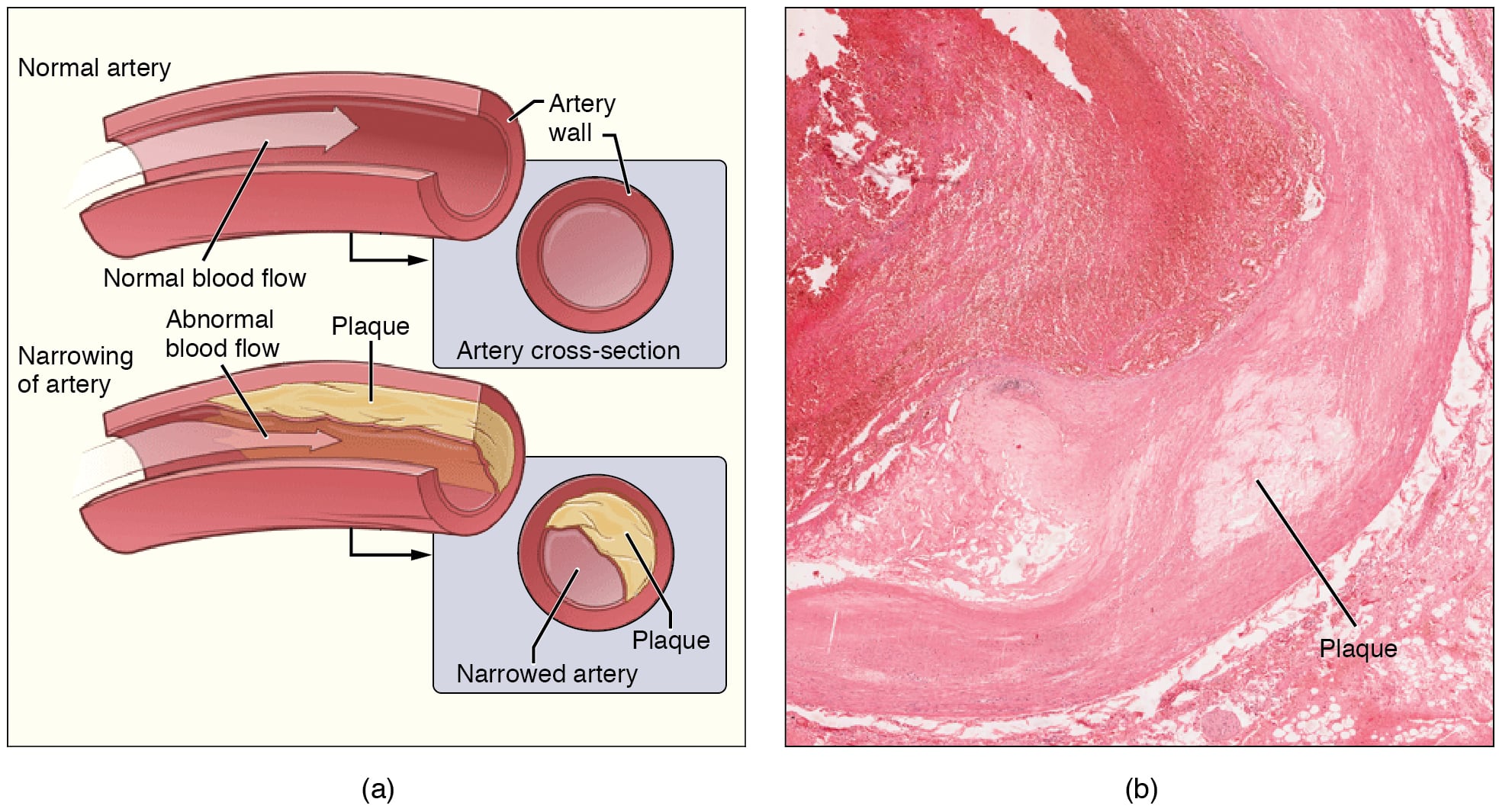
III) Atherosclerosis: Affects large and medium muscular arteries. It’s characterized by the formation of atheromas. It most commonly affects the aorta, coronary arteries, and cerebral arteries.
Major risk factors are HT, DM, smoking, dyslipidemias, hyperhomocysteinemia, obesity, C.pneumoniae, CMV, Herpes virus infections, stress, physical inactivity, trans fats, male sex, postmenopausal females, familial predisposition etc.
Pathogenesis: The initial trigger for atherosclerosis is endothelial injury. This can occur due to:
At sites of endothelial injury, platelet plugs form. Activated platelets and inflammatory cells (such as macrophages) release cytokines such as IL 1 and TNF alpha, which promote smooth muscle proliferation. This is further amplified by PDGF, TGF beta, FGF, Gamma Interferon, and endothelins, which stimulate smooth muscle proliferation and synthesis of matrix proteins such as collagen and proteoglycans.
Smooth muscle cells then migrate from the tunica media to the subendothelial space, where they proliferate in response to these growth factors.
Oxidized LDL plays a key role in plaque development. It downregulates ABCA 1 (ATP binding cassette transporter 1), which is involved in active efflux of cholesterol. Oxidized LDL is also pro-inflammatory: it recruits monocytes to the endothelium and promotes endothelial injury through free radicals. It enters the endothelial cell by binding to the LOX 1 receptor.
Reactive oxygen species and reactive nitrogen species oxidize LDL. Oxidized LDL is taken up by macrophages, leading to accumulation of cholesterol. Lipid-laden macrophages are called foam cells. Cholesterol is also deposited in smooth muscle cells.
Oxidized LDL also causes endothelial dysfunction by decreasing nitric oxide synthesis by the endothelium.
Collections of foam cells and fat-laden smooth muscle cells form yellow fatty streaks on the intima. These are the precursors to atheromatous plaques.
Plaques have:
Calcification, ulceration, and thrombosis of ruptured plaques can cause acute ischemia and infarction of the affected area, leading to AMI, cerebral stroke, etc.
Sign up for free to take 2 quiz questions on this topic