Retroviruses are RNA viruses that carry the enzyme reverse transcriptase. This enzyme converts viral RNA into DNA, which then integrates into the host cell genome. HIV and HTLV (Human T cell Lymphotropic Virus) are the two medically important retroviruses.
This group has 4 viruses, HTLV 1 to 4. HTLV 1 is the major pathogen, while not much is known about HTLV 2. HTLV 1 is endemic in South America, Africa, the Middle East, Japan, and the Caribbean. HTLV 3 and 4 do not cause any significant human pathology.
Morphology: HTLV is an enveloped virus with two copies of single-stranded RNA with positive polarity. The inner membrane of the virion envelope is lined by the viral matrix protein (MA). This structure encloses the viral capsid (CA), which carries two identical strands of the genomic RNA as well as functional protease (Pro), integrase (IN), and reverse transcriptase (RT) enzymes. gag, pol, and env as well as tax and rex are the important genes. The envelope proteins are gp46 and gp21.
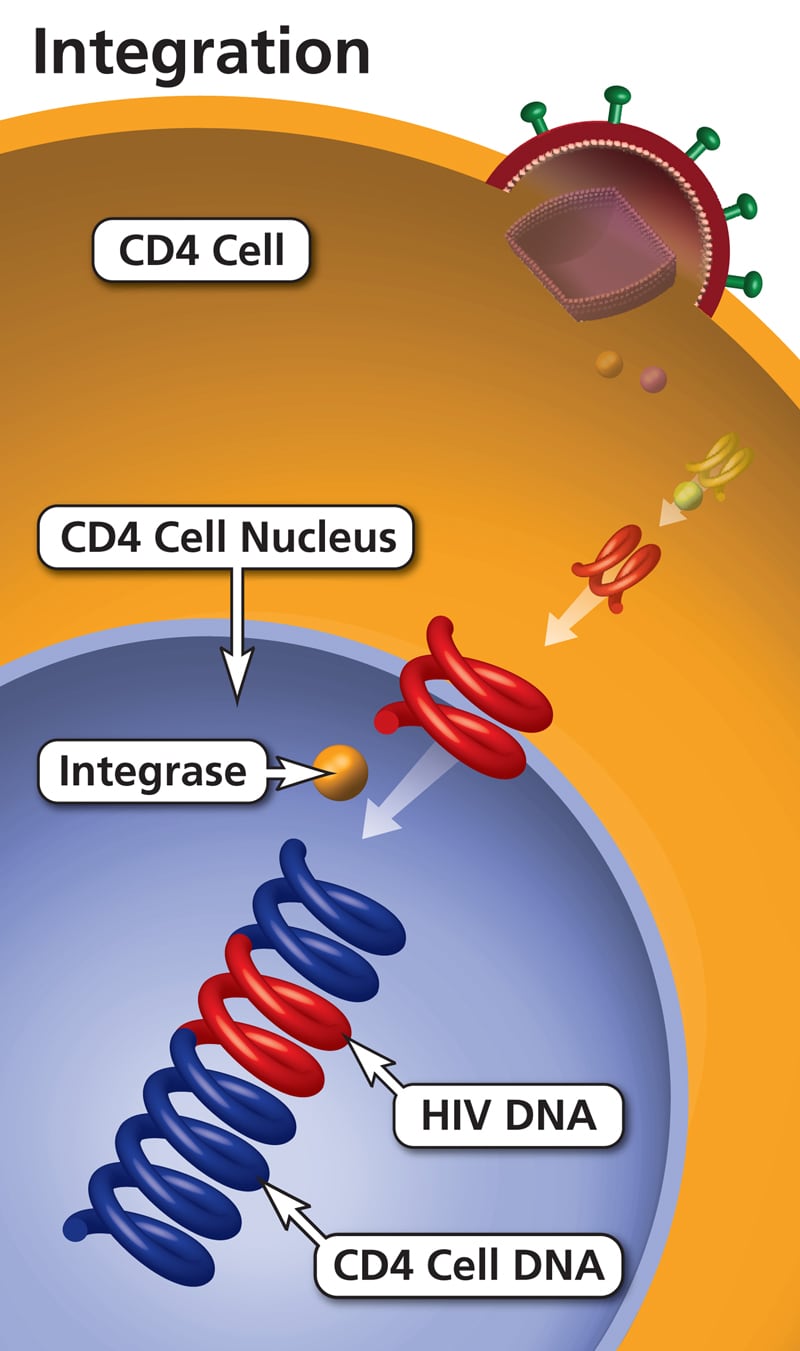
Pathogenesis: A newly synthesized viral particle attaches to the target cell receptor through the viral envelope (Env) and enters via fusion. This is followed by uncoating of the capsid and release of its contents into the cytoplasm. The viral RNA is reverse transcribed into double-stranded DNA by RT. This double-stranded DNA is then transported to the nucleus and integrated into the host chromosome, forming the provirus.
Tax and rex are regulatory genes. Tax is oncogenic. HTLV-1 basic leucine zipper factor (HBZ) interacts with cellular factors JunB, c-Jun, JunD, cAMP response element binding (CREB), and CREB binding protein (CBP)/p300 to modulate both viral and cellular gene transcription. Increased transcription and IL 2-mediated T cell growth lead to oncogenesis.
Clinical features: Transmission occurs via blood and blood products and by sexual intercourse. HTLV infects CD4+ T cells and causes them to proliferate.
It causes a progressive neurologic disease named HTLV-I associated myelopathy/tropical spastic paraparesis (HAM/TSP). Initial symptoms are subtle and include gait problems, unexplained falls, low back pain, constipation, urinary urgency/incontinence, and numbness or pain in the lower limbs. Symptoms progressively worsen over years.
Approximately 2-5% of HTLV-I carriers develop acute T cell leukemia/lymphoma. It presents with skin and bone lesions, pulmonary infiltrates, elevation of serum calcium, enlargement of the liver, spleen, and lymph nodes, and opportunistic infections.
HTLV 1 may also cause arthropathy, uveitis (inflammation of the eye), pneumonitis, and thyroid problems. HTLV 2 is associated with spinocerebellar syndrome and is seen in injection drug users.
Laboratory diagnosis of HTLV infections:
ELISA is used to detect antibodies, while Western Blot is used as a confirmatory test. PCR is used to detect viral RNA. Presence of HTLV antibody in CSF confirms HAM/TSP.
The earliest known case of infection with HIV-1 in a human was detected in a blood sample collected in 1959 from a man in Kinshasa, Democratic Republic of the Congo. On June 5, 1981, the first cases of AIDS presenting as Pneumocystis pneumonia were reported in the USA. It has been postulated that human HIV originated from African chimpanzees.
Morphology: HIV is structurally similar to HTLV, with a few antigenic differences. The genome is diploid, meaning it consists of 2 strands of positive-sense RNA. It is enveloped and icosahedral. The envelope has glycoproteins gp120 and gp41, which play a vital role in pathogenicity.
Along with the genes gag, pol, and env that encode structural proteins, HIV has 6 regulatory genes: tat and rev (both required for replication) and nef, vif, vpr, and vpu (accessory genes).
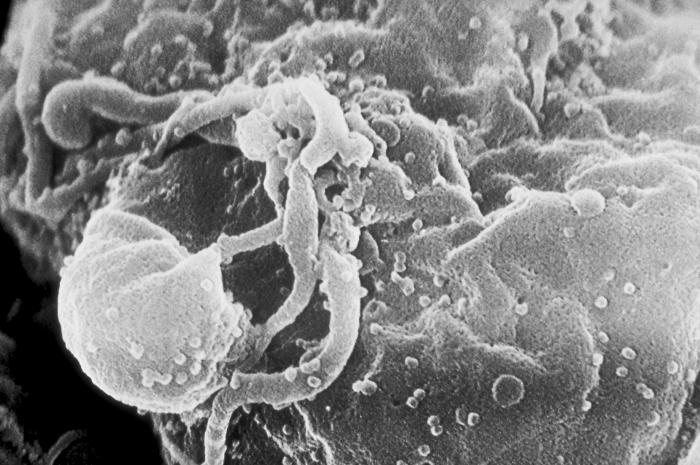
Scanning electron microscopic (SEM) image of human immunodeficiency virus-1 (HIV-1) virions seen as small round bumps, in the process of budding from a cultured lymphocyte.
Following is a short description of the HIV genes.
Classification of HIV: HIV-1 is one of the most polymorphic viruses known and exists as a swarm of genetically related variants (quasispecies). It frequently shows antigenic variation in the envelope antigen.
HIV has two antigenic types: HIV 1 and HIV 2. Type 1 is more common worldwide, while type 2 is seen mainly in West Africa. Type 1 has 10 subtypes (A to J), which are all included in Group M (Major). Subtype B is most common in the USA. Subtypes that do not fall into Group M form Group O (outlier). Newly discovered subtypes that do not fall in Groups M and O form Group N (new).
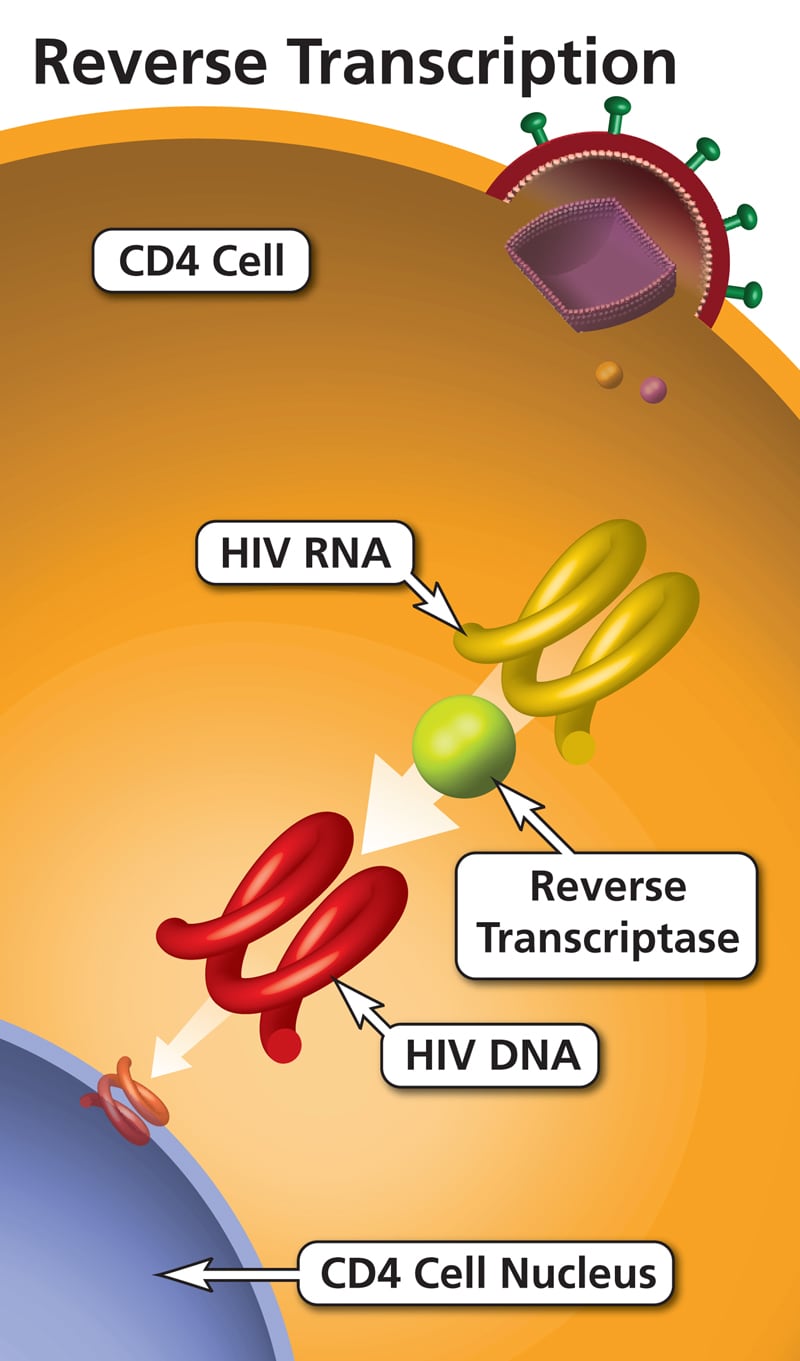
Pathogenesis: HIV infection typically begins without symptoms and is accompanied by slight changes in the immune system. This stage can span up to three months after infection, until seroconversion (when HIV-specific antibodies become detectable after recent exposure). It then takes several years for full-blown manifestations of AIDS.
During primary infection, the virus actively replicates in the lymph nodes and bloodstream. Individuals develop an AIDS status when their plasma HIV load is high and the CD4+ T count is less than 200/mm3.
HIV attaches to CD4+ cells and kills CD4+ T cells. It also attaches to chemokine receptors on monocytes and dendritic cells and kills them similarly. CCR5 and CXCR4 are the important chemokine receptors for HIV.
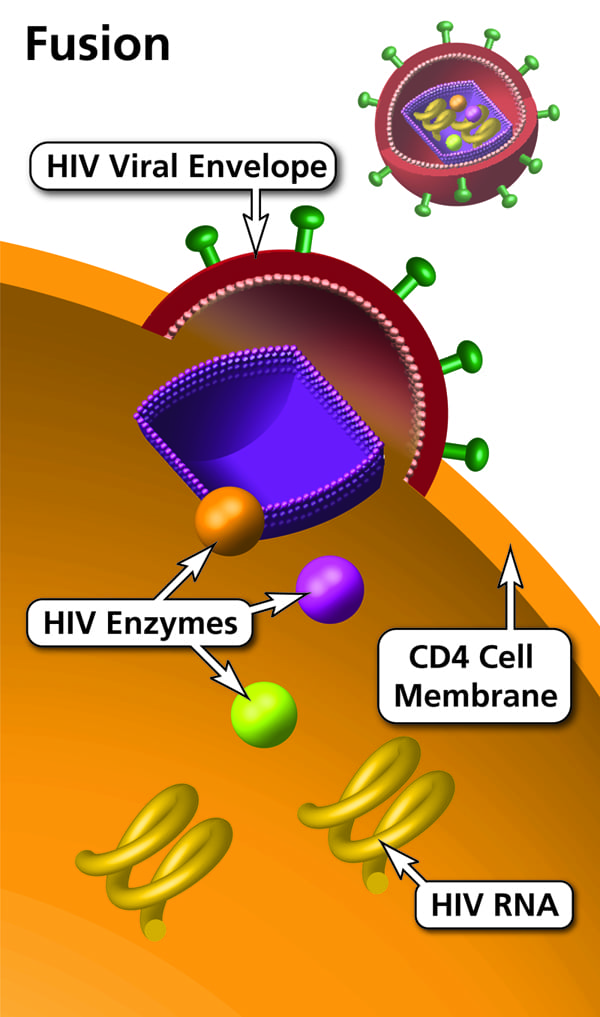
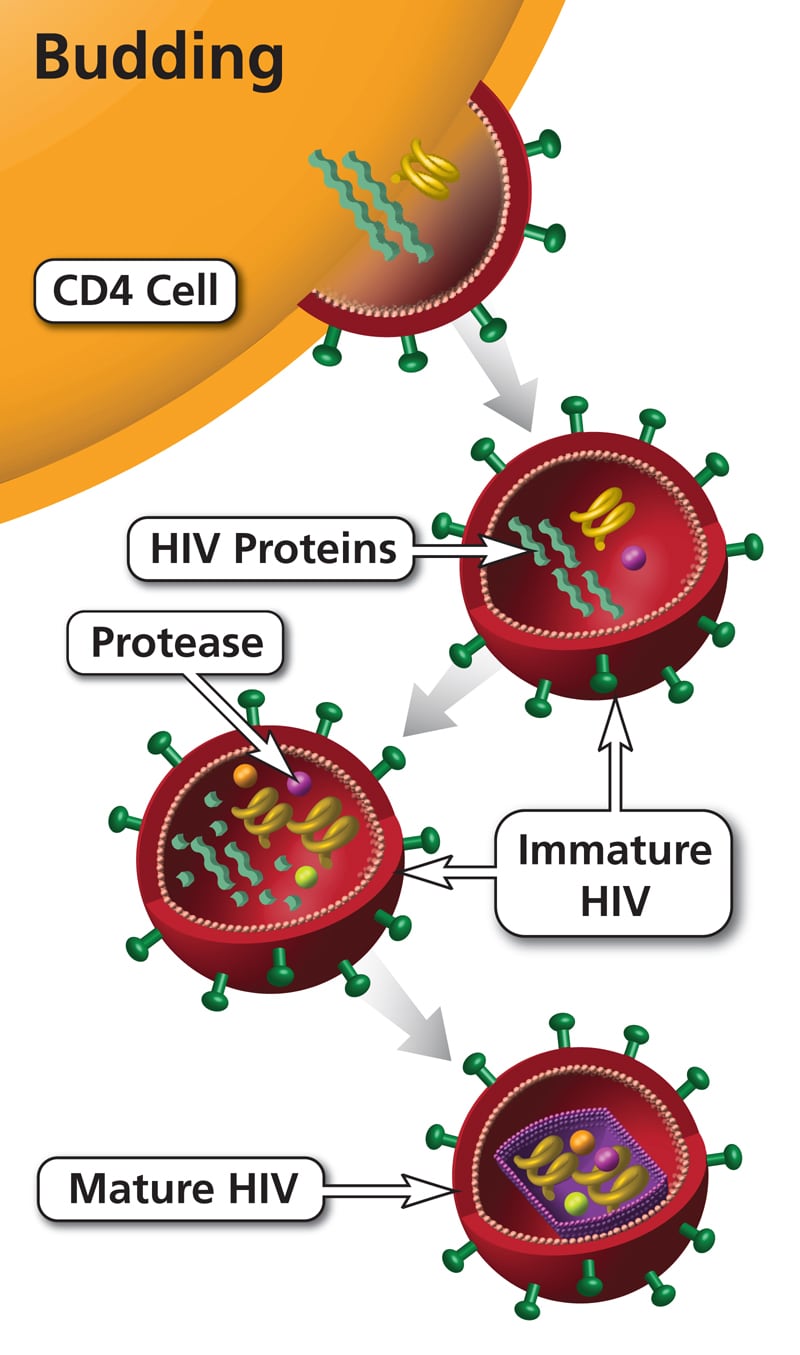
Macrophages and primary T lymphocytes express CCR5 and CXCR4, whereas T cell lines express only CXCR4. HIV can show tropism for macrophages, T cell lines, or both (dual tropism).
Macrophage (M)-tropic HIV-1 strains infect macrophages and lymphocytes using CCR5, while T-cell line (T)-tropic strains infect lymphocytes and T cell lines (but not macrophages) using CXCR4. Dual-tropic strains can infect all of these cell types. Other chemokine receptors, principally CCR3 and CCR2b, function as minor HIV coreceptors.
People who are homozygous for a mutant form of the CCR5 gene lack this receptor on their immune cells and are resistant to infection with some strains of HIV. Heterozygotes for the same mutation show slower progression of HIV.
Monocytes/macrophages are believed to serve as vehicles for dissemination of HIV between different tissues. Upregulation of viral coreceptors, activation of NF-kB (a transcription factor), and production of TNF-alpha (leading to induction of HIV replication in infected macrophages) help drive progression to AIDS.
Many cytokines such as TNF-alpha and -beta, IL-1, IL-2, IL-3, IL-12, MCSF, GMCSF, and IL-6 can enhance HIV replication and also play a key role in AIDS-associated malignancies. In individuals infected with HIV, the normal Th1 response to viral infection is shifted to a Th2 response.
Death of HIV-infected CD4+ cells is attributed to:
Clinical features: Transmission is by sexual contact, blood and blood products, perinatal transmission, and through breast feeding. Infection goes through the following three stages.
Acute HIV Infection / Seroconversion Illness: 2-4 weeks after exposure, patients present with a flu-like illness with fever, chills, malaise, fatigue, night sweats, lymphadenopathy, and myalgia. Symptoms usually resolve in a few days. Some people may be asymptomatic.
Clinical Latency: A stage of chronic infection that may persist for 10-15 years. Patients are typically asymptomatic, though the virus is actively multiplying in the body.
AIDS or Acquired Immunodeficiency Syndrome: Severe symptoms manifest as extreme fatigue, weight loss, cognitive disturbances, pneumonia, generalised lymphadenopathy, chronic diarrhea, etc. These symptoms are due to the onset of opportunistic infections.
Laboratory diagnosis of HIV infection: Diagnosis is guided by the stage of infection.
Presumptive diagnosis is done by ELISA and must be confirmed with Western Blot. Rapid tests based on immunoblot assay, immunochromatography, and particle agglutination (using latex, gelatin, or RBCs) give results in 20 minutes but must be confirmed with Western Blot.
A positive Western Blot shows at least two bands: p24, gp41, and/or gp120/160.
Nucleic acid detection can be done by PCR, RT-PCR, or in situ hybridisation tests. RT-PCR is used to determine viral load. For research purposes, virus can be isolated by co-cultivation of a patient’s lymphocytes with uninfected lymphocytes in the presence of IL 2.
Antibodies (IgM or IgG) can be detected by ELISA. p24 antigen detection can also be done by ELISA.
Nucleic acid assays will be positive in all stages of infection. p24 antigen assays become negative once antibodies are detectable.
In newborns born to HIV-positive mothers, diagnosis is established by DNA/RNA PCR, p24 antigen assay, or viral culture.
Window period: The window period refers to the time after infection and before seroconversion, during which markers of infection (p24 antigen and antibodies) are absent or too scarce to be detectable. It may vary from a few weeks to not more than 90 days. Viral culture and PCR will still be positive in the window period.
Acute Illness: Antibodies may be absent because they take at least 2 weeks to appear. Diagnosis in this stage is done by RT PCR, p24 antigen assay, or viral culture. When antibodies develop, ELISA will be positive, with IgM appearing first followed by IgG.
Sign up for free to take 2 quiz questions on this topic