Knowing the conversions is important when using formulas and calculations while compounding in pharmacy. Following are the important conversions to know
Following are the Roman numerals and their corresponding numbers.
Concentration is the amount of active ingredient per total substance weight. Liquids are expressed as weight/volume (), with the weight being the amount of drug and the volume representing a specific volume of drug and vehicle. For example, if the concentration of a suspension is , that means there is mg of the active drug in of the suspension. Solid topical medication concentrations are usually expressed as weight/weight (), with the numerator representing the weight (mass) of the drug present in the denominator, which is the total weight of the drug plus vehicle. For example, nystatin cream is available in a concentration of . This means that each gram of cream has units of nystatin. If the concentration of a liquid medication is stated as (), e.g., , then it has of the active drug in of the total liquid.
Specific gravity refers to the ratio of the weight of a substance to the weight of an equal volume of water at the same time. If the specific gravity is known, the volume or weight of the desired quantity can be determined.
Specific gravity
Number of tablets
The above formula can be used when the prescription strength is not in stock, e.g., if the prescription states to dispense of drug A, but you only have available.
Number of tablets of drug A tablets.
Hence, you can dispense four tablets of each to replace one tablet of .
Amount of solution to be given
The above formula can be used when calculating the volume of a liquid medication to be dispensed, assuming the strength or concentration of the stock solution is known. For example, if the stock solution has a strength of and you need to dispense , how much volume of the stock solution will you dispense?
Using the formula above, the amount of solution to be given of stock solution needs to be dispensed.
Ratios are a way to express the strength of a solution or liquid preparation. In ratio strength, the first number is a , and a colon follows it and then another number, e.g., . The units are always grams or milliliters, depending upon whether you are dealing with a or preparation. Thus, a ratio strength means a solution with g in ml or a solid preparation, say g of drug in g of ointment. For example, a ratio for epinephrine represents gram of epinephrine in ml of solution, so the amount per unit of volume is . A ratio for epinephrine represents gram of epinephrine in ml of solution, so the amount per unit volume is .
Percent strength: Percent strength represents the number of grams contained in of product.
Percent weight in volume (w/v): The number of grams in of solution is expressed as . Powdered substances suspended in a liquid vehicle would be calculated as . For example, solution will have 1 gram of the powder in of the solution.
Percent volume in volume (v/v): The number of milliliters in of solution and is expressed as . A liquid component in a liquid preparation would be calculated on a basis.
Percent weight in weight (w/w): The number of grams in grams of total dosage form and is expressed as . Powdered substances mixed with a solid or semisolid would be calculated as , e.g., ointments.
If a solution requires a dilution, the active drug in the solution will remain constant, but the volume will increase. When two solutions have equal osmotic pressure and salt concentration, they are said to be isotonic. Normal saline has a concentration of of NaCl in sterile water and, therefore, is an isotonic crystalloid.
Calculating drug dosage based on body surface area: In some conditions, drug dosage is calculated according to body surface area (BSA).
, where is body weight in kg, is body height in meters
Pediatric dosing: Clark’s and Young’s rules are used to calculate drug dosages in the pediatric age group ( from birth to about 18 years of age).
According to Clark’s rule, the pediatric dose is obtained by dividing the patient’s weight in pounds by the average standard weight of pounds multiplied by the adult dose of a drug.
Young’s rule uses the patient’s age to calculate the pediatric dosage.
Fried’s rule also uses age to calculate the pediatric dosage.
Pediatric doses are often stated as per body weight, e.g., .
Estimating intravenous (IV) flow rates: Intravenous infusions administer fluids directly into the veins. The rate of infusion can be calculated in milliliters or drops.
Drops per minute are abbreviated as gtts/min. The drop factor is the number of drops in . The width of the tube used for administering the infusion determines the size of the drop. Macrodrip tubing administers a larger drop and may be used for , , or . Macrodrips are used when rapidly infusing large amounts of fluids. Microdrip tubing administers . Microdrips are typically used in children. All IV packages clearly label the gtts/mL for that set.
The following formulae can be used to set the rate of the IV infusion:
Total IV volume/time (hour or minute)
International units (IU): International units are used to denote doses for hormones like insulin, vaccines, vitamins, blood products, etc. They measure the drug’s effect or biological activity, not its weight or mass.
Milliequivalent (mEq): The milliequivalent (mEq) is the unit of measure often used for electrolytes. It indicates the chemical activity, or combining power, of an element relative to the activity of of hydrogen.
Alligation: The method of mixing two liquids or solids of different concentrations to produce a mixture of the desired concentrations. The end product has a different concentration than the original. We need to mix a specific proportion of each ingredient to get the desired concentration, which is given by the alligation ratio.
Considering “m” as the desired concentration, ‘l’ is the lower concentration, and “h” is the higher concentration.
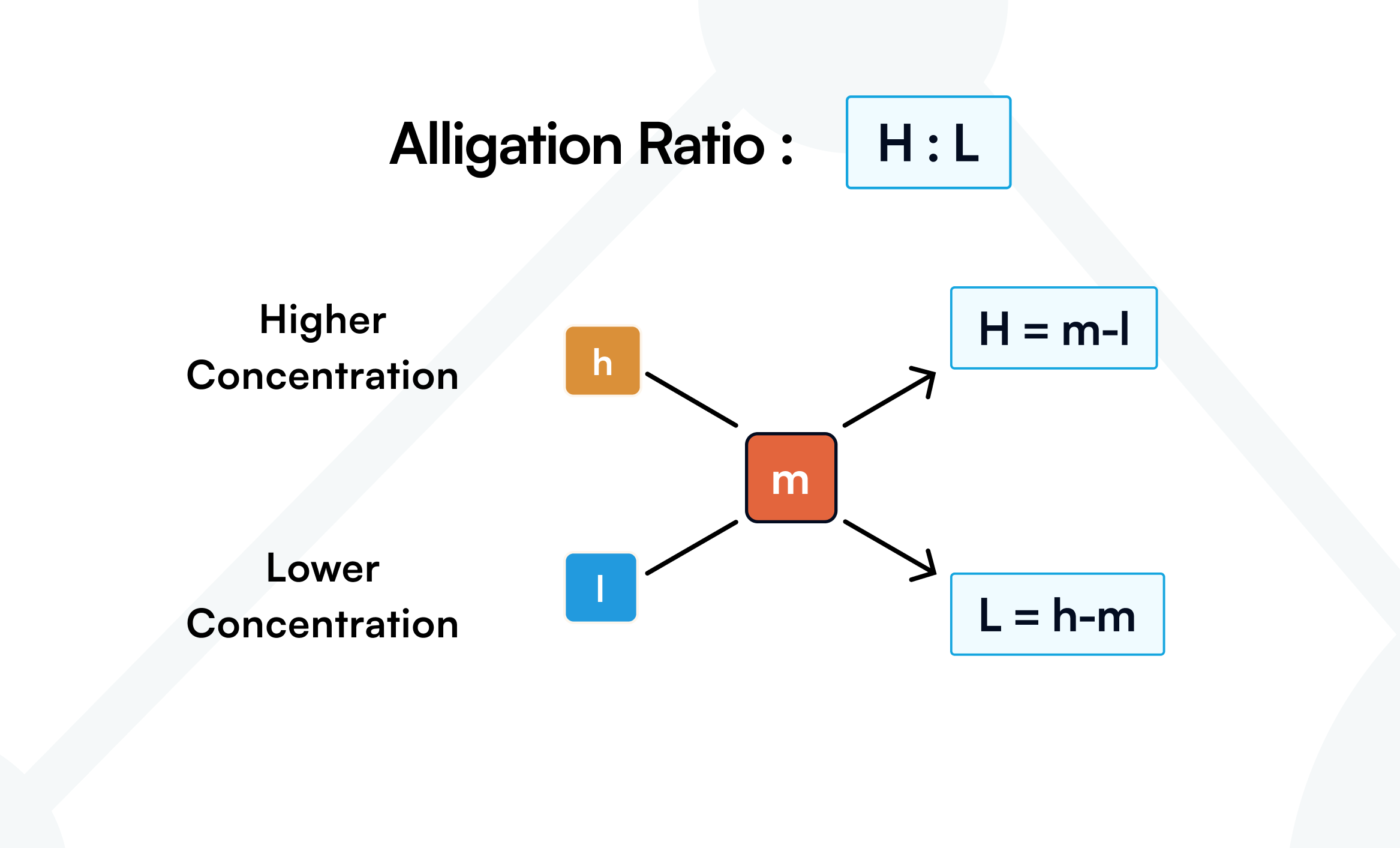
Once the alligation ratio is known, e.g., , the proportion is two parts of higher concentration to three parts of lower concentration. This is followed by calculating the alligation volume, which is simply converting the parts into volume. For example, if we are compounding of the final solution, then a ratio of corresponds to of solution of higher concentration and of solution of lower concentration.
For example, an order comes in for grams of hydrocortisone cream. You only have and hydrocortisone available. Using the alligation method,
Hence, alligation ratio = .
You need to mix equal parts of and to get concentration. As we need grams, mix grams of hydrocortisone with grams of hydrocortisone to get grams of hydrocortisone.
In another example, suppose you get an order for of a solution with a concentration. You have and concentrations available. How will you use the alligation method to calculate the volume of each concentration required?
Alligation ratio =
We need two parts of higher concentration and five parts of lower concentration . There are many ways to get the volume. You can use the following equation -
Hence, mix of and of to get of .
Dilution: Dilution is a method of preparing a solution of desired concentration by diluting a concentrated stock solution. Water is typically used to dilute the solution. Dilution can be done by using the following formula:
where and are equal to the molarity of the solutions, measured as mol/L or M, and and are equal to the volume of the solutions. Sometimes, concentration is given in or concentrations. In that case substitute and by the respective concentrations.
How much water should you add to of a solution to reduce its strength to a solution?
Using the formula above,
Since we already have of the solution, subtract that from .
of water needs to be added to of solution to get a solution. Make sure to keep the same unit system of measurement while doing calculations. For example, if using volume in “ml,” keep it the same on both sides of the equation by converting “L” to “ml.”
Aliquot: The dilution method used when a minimal quantity of the drug is required. It is used when the minimum measurable or weighable quantity (MMQ or MWQ) of the equipment available is much higher than the quantity of drug needed. For example, if MWQ is and the amount of drug required is .
In the above example, measure out the MWQ of and dilute it to a desirable dilution, e.g., you can dilute with of water to make a solution. Measure of the solution to get of the drug.
Sign up for free to take 20 quiz questions on this topic