Pharmacokinetics is “what the body does to the drug”. This includes absorption, distribution, metabolism, excretion, and drug elimination.
“ADME” principle for the levels of pharmacokinetic interactions -
“A” for Absorption
“D” for Distribution
“M” for Metabolism
“E” for Elimination
The absorption of a drug is determined by the route of administration and properties of the drug itself. Small, unionized, lipid-soluble drugs are absorbed easily. Most drugs are absorbed in the small intestines because of their large surface area. Most drugs are absorbed by passive diffusion, i.e., they move from an area of high concentration to an area of low concentration. Other drugs are absorbed by active transport, facilitated passive diffusion, and pinocytosis. Weak acids will be unionized in an acidic environment and hence absorbed better in such an environment. Weak bases are unionized in an alkaline environment and are absorbed better in an alkaline environment. For the same reason, alkalinizing the urine can accelerate the excretion of weak acids like aspirin and vice versa.
The distribution of a drug within the body is not uniform, as various factors influence its concentration in different tissues. A drug’s solubility, for instance, plays a crucial role; lipid-soluble drugs, such as certain anesthetics, can readily cross into lipid-rich areas like the brain. The degree of tissue binding also affects distribution, with drugs like warfarin predominantly remaining in the bloodstream due to their strong binding affinity to plasma albumin. Moreover, the rate at which a drug accumulates in tissue is significantly influenced by the blood supply to the area, with well-perfused tissues experiencing faster concentration increases compared to those with less blood flow. Additionally, the overall mass of the tissue can impact drug absorption. The nature of the drug itself dictates its binding preferences; acidic drugs generally have a higher affinity for albumin, while basic drugs tend to bind more extensively to proteins such as alpha-1 acid glycoprotein and lipoproteins. This binding not only determines the drug’s distribution but also extends its duration of action, highlighting the complex interplay of factors that influence drug distribution within the body.
The volume of distribution, or Vd, measures a drug’s distribution in the body. Drugs with a small Vd are confined to the blood, while drugs with a large Vd are distributed extensively throughout the various body compartments and tissues.
Occurs primarily in the liver. Other sites are the kidneys, lungs, blood, and intestines. Prodrugs are metabolized to form active drugs. Most drugs become inactive after metabolism. Some drugs may produce toxic products during their metabolism. An essential part of metabolic reactions is to make the drug or its products more water soluble, i.e., polar so that it can be easily eliminated from the body.
Types of metabolic reactions:
Phase 1 reactions (non-synthetic): Converts the drug to a more polar or more reactive/active form. Oxidation, reduction, deamination, hydrolysis.
Phase 2 reactions (synthetic): Make the drug more water soluble; the end product is inactive. Conjugation with glucuronic acid, glycine, sulfate, glutathione, etc.
Drug metabolism rates vary among patients. In rapid metabolizers, effective blood and tissue levels of the drug may not be reached, while in slow metabolizers, the risk of adverse effects is higher. The rate of metabolism is affected by genetics, age, coexisting liver or other diseases, and concomitant use of drugs affecting metabolism.
Cytochrome P450 enzymes, located primarily on the smooth endoplasmic reticulum of the liver, carry out most oxidation reactions. There are approximately 60 cytochrome P450 genes in humans. Polymorphisms in Cyt P450 enzymes affect drug metabolism. The major ones are CYP3A4 and CYP2D6. Inhibitors of Cyt P450 decrease the enzyme’s activity, while inducers increase it.
Common inhibitors and inducers of Cyt P450 enzymes:
Inhibitors: Cimetidine, amiodarone, erythromycin, ticlopidine, ciprofloxacin, omeprazole, fluconazole, bupropion, metoclopramide, quinidine, disulfiram, metronidazole, grapefruit juice, ritonavir, saquinavir. Need to lower the dose of a drug given concomitantly with any drug (s) in this group.
Inducers: Carbamazepine, phenytoin, rifampin, phenobarbital, isoniazid, alcohol, tobacco, dexamethasone, prednisone. Need to increase the dose of a drug given concomitantly with any drug (s) in this group.
Most drugs are excreted by the kidney, inhaled anesthetics are mainly excreted by the lungs, and some drugs are excreted in bile and feces. Elimination converts a drug to an inactive metabolite, which terminates drug activity. Drugs undergo two types of elimination: first-order and second-order.
First-order elimination occurs when the rate of elimination is directly proportional to the remaining concentration of the drug, i.e., a constant fraction of the drug is eliminated per unit time. Such drugs have a constant half-life of elimination. When plasma concentration is plotted against time, they show an exponential decrease in plasma concentration. Drug concentration will decrease by 50% for every half-life. Most drugs follow first-order kinetics.
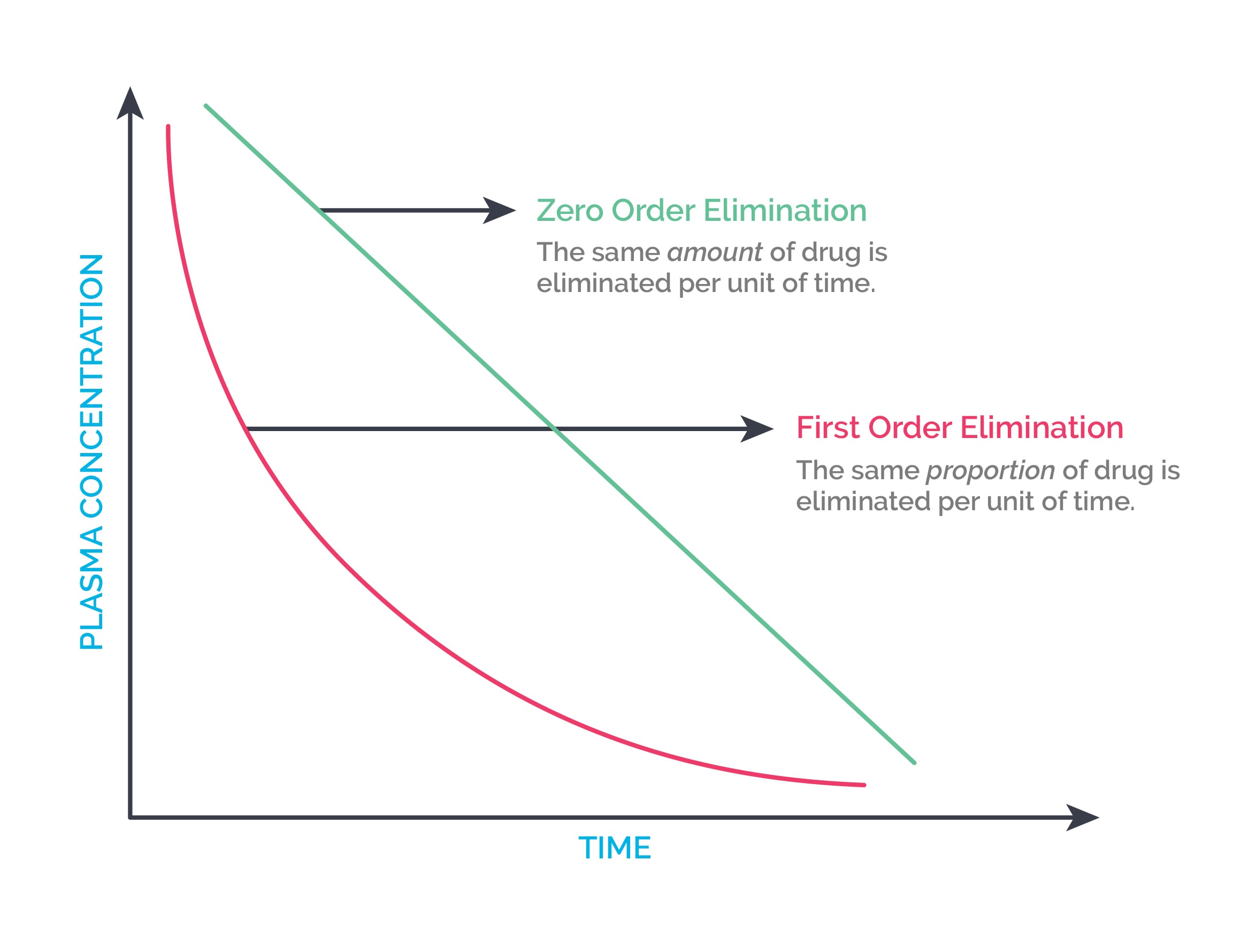
Zero-order elimination occurs when a constant quantity of the drug is eliminated per unit of time. Drugs that saturate their elimination mechanisms show zero-order kinetics. When plasma concentration is plotted against time, it shows a linear decrease. It does not have a constant half-life. Ethanol, aspirin, phenytoin at high doses, omeprazole, fluoxetine, and cisplatin show zero-order elimination.
| Example | ADME level | Mechanism |
| (Tetracyclines or quinolones) + multivalent cations (calcium, magnesium, or aluminum) | Absorption | Reduces absorption by the formation of complexes that cannot be absorbed |
| Itraconazole + antacids | Absorption | Increased pH minimizes the absorption of acidic drugs |
| Cyclosporine + St. John’s wort | Absorption | St. John’s wort increases the activity of P-glycoprotein* in the intestinal cells and decreases cyclosporine levels |
| Phenytoin + valproic acid | Distribution | Both drugs compete for binding sites on albumin |
| Rifampin + oral contraceptive pills | Metabolism | Rifampin induces Cyt P450, causing decreased estrogen levels |
| Warfarin + grapefruit juice | Metabolism | Grapefruit juice inhibits Cyt P450, causing increased warfarin levels, which may cause bleeding |
| Penicillin + probenecid | Elimination | Increased penicillin levels as probenecid competes with penicillin for transporters in the kidney |
*P-glycoprotein is a membrane transporter that transports substances out of the cell. Certain drugs, supplements, and foods activate or inhibit P-glycoprotein, which can decrease or increase the levels of medications taken along with them.
Sign up for free to take 13 quiz questions on this topic