Covalent bonds form when two atoms share electrons, resulting in the overlap of their electron orbitals. This sharing enables the atoms to achieve stability by filling their valence shells.
In covalent bonding, the first bond formed between two atoms is a sigma () bond, which occurs through direct orbital overlap along the internuclear axis. In molecules with multiple bonds, the additional bonds are pi () bonds; for example, a double bond consists of one sigma bond and one pi bond, while a triple bond consists of one sigma bond and two pi bonds.
To explain molecular shapes, hybrid orbitals are formed by mixing standard atomic orbitals. Common types include:
The Valence Shell Electron Pair Repulsion (VSEPR) theory further explains molecular geometry by predicting that electron pairs around a central atom will arrange themselves as far apart as possible to minimize repulsion. This theory accounts for the three-dimensional shapes of molecules like (trigonal pyramidal), (bent), and (linear).
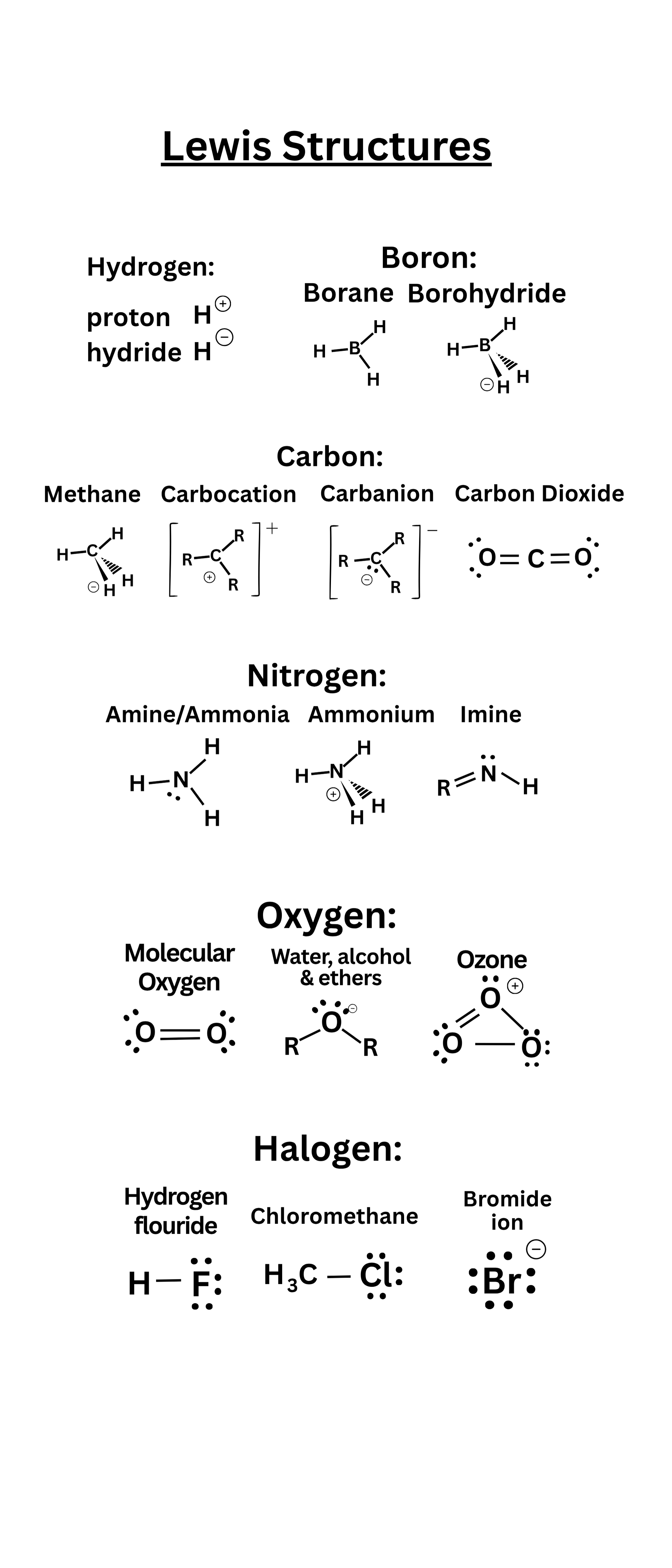
Lewis Electron Dot formulas are diagrams that use dots to represent valence electrons around atoms and lines to represent shared electrons in chemical bonds. In these structures, each dot stands for 1 electron, while each line stands for 1 shared pair of electrons (2 total).
Carbon ():
Typically 4 total bonds and no lone pairs.
Examples include (methane) or (carbon dioxide).
Oxygen ():
Nitrogen ():
Halogens (, , , ):
1 bond, 3 lone pairs in neutral forms.
Example: in .
Hydrogen ():
1 bond, 0 lone pairs.
Hydrogen is an exception to the octet rule because it only requires 2 electrons (a “duet”).
Carbocation ():
3 bonds, no lone pairs, overall +1 charge.
Carbanion ():
3 bonds, 1 lone pair, overall −1 charge.
Boron ():
3 bonds, 0 lone pairs (6 electrons total around B).
E.g., (borane).
Similarity across a group (column)
Lewis structures for elements in the same group share similar patterns. For instance, sulfur often appears in the same bonding patterns as oxygen, but it can expand its valence shell (e.g., in with 12 electrons around S) with 12 electrons around S). Thus, you can usually substitute S for O in many basic diagrams, keeping in mind sulfur’s ability to exceed the octet.
Lewis acids and bases
According to Lewis theory, a Lewis acid is an electron pair acceptor and a Lewis base is an electron pair donor. Unlike the Brønsted-Lowry concept that focuses on protons, the Lewis framework centers on electron pair movement. When a Lewis base donates its pair, a coordinate covalent bond forms, satisfying the acid’s electron deficiency. This mechanism underlies many reactions, such as metal-ligand complex formation, where an electron-deficient metal ion accepts electron pairs from the ligand.
Resonance structures
In both ions and molecules, resonance describes the phenomenon where electrons are not confined to a single bond or atom but instead are delocalized across multiple atoms. This explains why, when drawing resonance structures, electrons are depicted as being “pushed” to indicate their movement. In ions, this delocalization serves to distribute charge evenly, while in molecules it is most evident in systems containing aromatic rings and conjugated double bonds.
Resonance structures occur when a molecule can be represented by more than one acceptable Lewis structure. Rather than rapidly oscillating between these forms, the molecule exists as a hybrid that incorporates features of each structure, with a greater contribution from the more stable forms. For example, if one resonance form shows a single bond and another a double bond, the actual bond length will be intermediate between that of a typical single and a typical double bond.
In stable resonance forms, the octet rule is fulfilled for each atom (except for known exceptions like boron and hydrogen), and formal charges are minimized or arranged so that like charges are separated while opposite charges are positioned close together.
Partial ionic character
Partial ionic character occurs when covalent bonds form between atoms with different electronegativities. In these bonds, the atom with higher electronegativity draws electrons closer, acquiring a partial negative charge, while the other atom becomes partially positive.
This unequal distribution of charge leads to a dipole moment when the molecule’s shape is asymmetrical, making it polar. The magnitude of the dipole moment depends on both the extent of charge separation and the distance between the charges.
For instance, water () has a bent structure that results in a net dipole, whereas a molecule like , despite having polar bonds, has its charge distribution arranged symmetrically so that the individual dipoles cancel out, rendering the molecule non-polar.
σ and π bonds A (sigma) bond is formed by direct, head-on overlap of orbitals along the internuclear axis, creating a cylindrically symmetrical region of electron density between two atoms. A (pi) bond arises from the side-by-side overlap of p orbitals, resulting in electron density above and below (or in some cases in front of and behind) the internuclear axis. Together, these concepts explain how single, double, and triple bonds form in molecules containing H, C, N, O, F, S, P, Si, and Cl.
| Element | Valence e- | Usual # bonds | Typically found in |
|---|---|---|---|
| H | 1 | 1 | Hydrocarbon (alkane, alkene, alkyne), hydride. All organic compounds contain hydrogen. |
| C | 4 | 4 | Alkane, alkene, alkyne, aromatic rings. All organic compounds contain carbon. |
| N | 5 | 3 | Amine, amide, imine, hydrazone, oxime, nitro compound, diazo compound, nitrile/cyanide, azide |
| O | 6 | 2 | Alcohol, ether, aldehyde, ketone, carboxylic acid, acyl halide, anhydride, amide, ester, ozone |
| F | 7 | 1 | Fluoride |
| S | 6 | 2 or 6 | Thiol, sulfide, sulfate, sulfite |
| P | 5 | 3 or 5 | Phosphorous compound, phosphate, phosphite |
| Si | 4 | 4 | Silane, silicon dioxide |
| Cl | 7 | 1 | Chloride, hypochlorite, chlorite, chlorate, perchlorate |
Sign up for free to take 7 quiz questions on this topic