In the liquid phase, molecules are closely packed and experience significant intermolecular forces that influence properties like boiling point and viscosity. One of the key interactions in this phase is hydrogen bonding, a specific type of dipole-dipole interaction.
In hydrogen bonding, a partially positive hydrogen bond donor—a hydrogen atom bonded to highly electronegative atoms such as fluorine, oxygen, or nitrogen—attracts a partially negative hydrogen bond acceptor from a neighboring molecule. This interaction requires extra energy to break, thus increasing the boiling point of the substance. The strength of hydrogen bonds depends on the bond’s polarity, with
For example, ethers cannot form hydrogen bonds with one another because they lack a hydrogen atom attached to F, O, or N, and therefore do not have a donor necessary for hydrogen bonding.
Dipole-dipole interactions occur between polar molecules that align so that the positive end of one molecule attracts the negative end of another. This attractive force increases the boiling point of a substance, though it is generally less strong than hydrogen bonding. The strength of these interactions depends on how polar the molecules are.
In contrast, ion-dipole interactions involve a charged ion interacting with a polar molecule. Because one component carries a full charge rather than just a partial charge, these interactions are stronger than dipole-dipole forces. Their strength increases with a greater magnitude of the ion’s charge and a higher polarity in the dipole.
Van der Waals’ forces, also known as London dispersion forces, are a type of dispersion force that occurs in all molecules, though they are especially significant in non-polar molecules where other intermolecular attractions, such as dipole-dipole interactions, are minimal.
These forces arise from the formation of induced dipoles—when a polar molecule influences a nearby non-polar molecule to temporarily develop a dipole—and instantaneous dipoles, which are fleeting fluctuations in electron distribution within non-polar molecules that momentarily create dipoles. The strength of these forces increases with molecular size, meaning that larger molecules like decane () exhibit stronger dispersion forces than smaller molecules like ethane ().
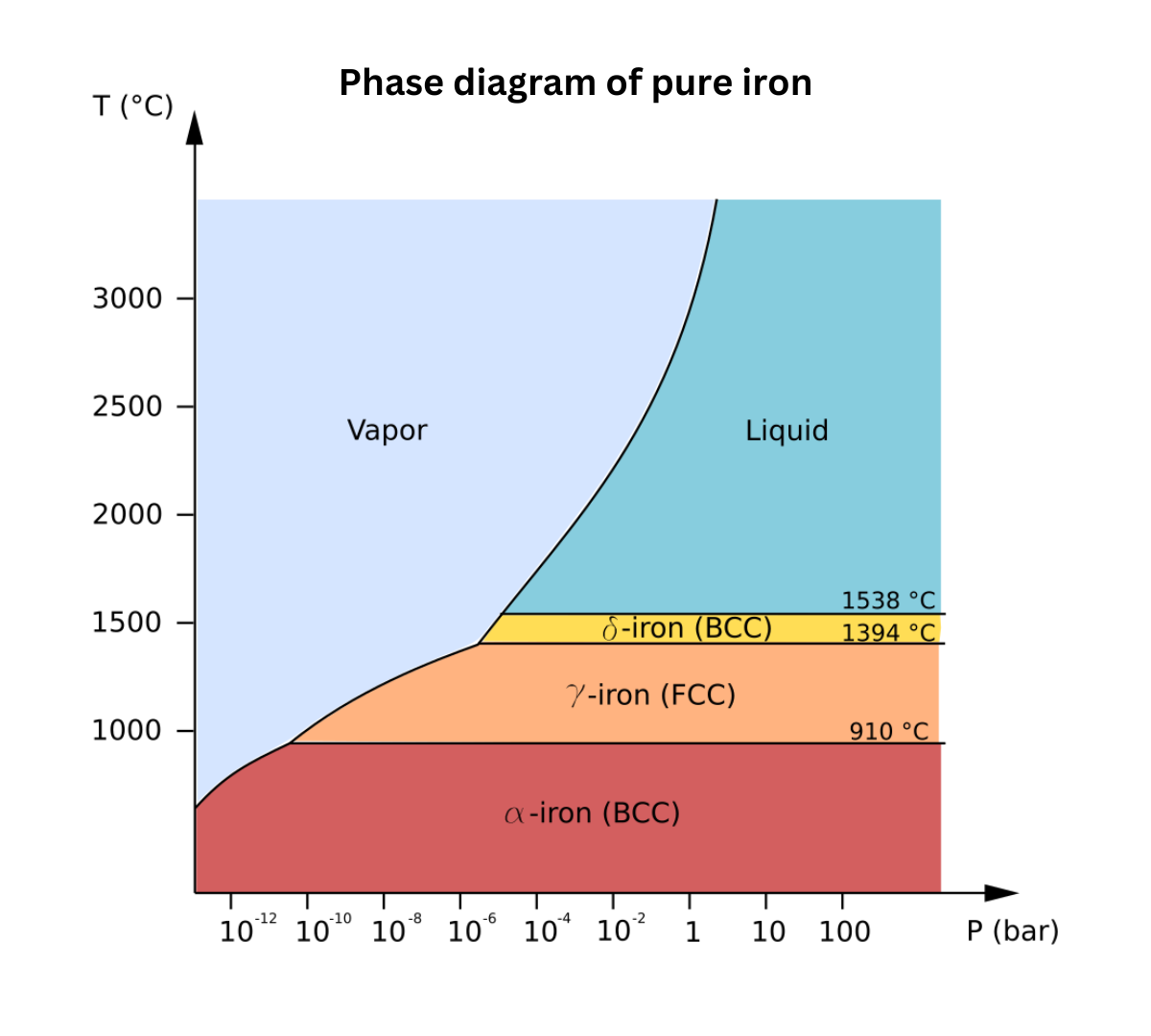
A phase diagram illustrates the conditions under which a substance exists as a solid, liquid, or gas. In the solid state, molecules vibrate around fixed positions, rendering the substance nearly incompressible and unable to flow. In the liquid state, molecules, though still close together due to intermolecular forces, can move past one another, allowing the liquid to flow and take the shape of its container while remaining largely incompressible. In contrast, in the gas state, molecules move randomly and are spaced far apart, making gases easily compressible and capable of filling any container.
The diagram also shows phase boundaries where two states coexist in equilibrium: the solid-liquid boundary represents equilibrium between solids and liquids, the solid-gas boundary between solids and gases, and the liquid-gas boundary between liquids and gases.
The triple point is the specific combination of temperature and pressure at which all three phases exist simultaneously
The critical point marks the conditions where the liquid and gas phases become indistinguishable
The critical temperature is the highest temperature at which a liquid can exist.
Notably, water’s phase diagram is unique because its solid-liquid boundary slopes to the left, reflecting that liquid water is denser than ice; hence, increasing pressure converts ice into water.
Freezing, melting, boiling, and sublimation
Colligative properties are characteristics of a solution that depend solely on the number of solute particles present, regardless of their specific identity. The presence of these solute particles helps maintain the liquid phase, which makes it more difficult for the liquid to boil (raising its boiling point) and harder to freeze (lowering its freezing point). In essence, reducing the vapor pressure of a solution is equivalent to elevating its boiling point.
A critical concept in colligative properties is the van’t Hoff factor (i), which accounts for the total number of particles in solution. For instance, a solute like glucose, which does not dissociate, has a van’t Hoff factor of 1, whereas a solute like , which separates into and ions in solution, has a van’t Hoff’t factor of 2.
The phenomenon of vapor pressure lowering is described by Raoult’s law, which states that the vapor pressure of a solution is equal to the product of the mole fraction of the solvent and the vapor pressure of the pure solvent , or
Conversely, the decrease in vapor pressure can be expressed as
where is the mole fraction of the solute.
Boiling point elevation is quantified by the equation
where is the increase in the boiling point, is the molal boiling point constant, is the molality (moles of solute per kilogram of solvent), and is the van’t Hoff factor.
Similarly, freezing point depression is described by
where represents the decrease in the freezing point (the negative sign indicates a reduction), is the molal freezing point constant, is the molality, and is the van’t Hoff factor.
Osmotic pressure () is another important colligative property, defined as the pressure required to prevent the movement of solvent across a semi-permeable membrane from a region of low solute concentration to a region of high solute concentration. It is given by the formula
where M is the molarity of the solution (in mol/L), R is the ideal gas constant, T is the absolute temperature in Kelvin, and is the van’t Hoff factor. Osmotic pressure determines the direction and extent of osmosis.
Differentiating types of mixtures:
Sign up for free to take 8 quiz questions on this topic