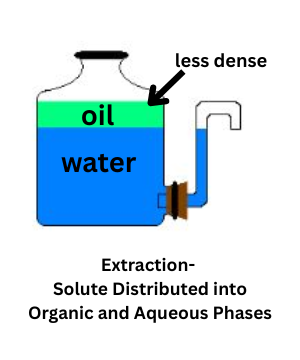
Extraction is a process used to separate a solute between two immiscible liquids. Typically performed in a separatory funnel, this method relies on the difference in solubility of the solute in the two layers: the organic phase and the aqueous phase. The organic phase is composed of a nonpolar solvent where nonpolar solutes dissolve, whereas the aqueous phase, usually water, dissolves ionic and polar compounds. The relative density of these solvents determines their positioning; while water is generally denser than many organic solvents, certain solvents like chloroform are denser than water and will settle at the bottom.
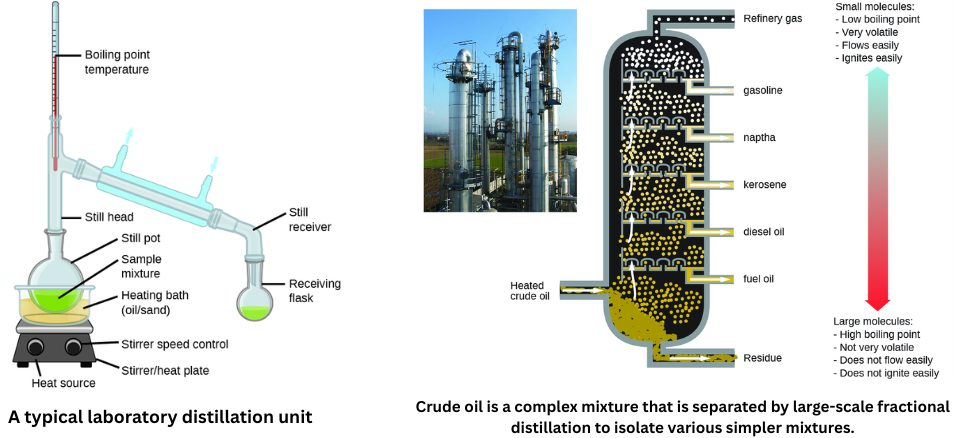
Distillation is a separation technique that exploits differences in boiling point and vapor pressure between liquid components. In a mixture of two volatile liquids, the one with a lower boiling point vaporizes more readily. According to Raoult’s law, the vapor phase becomes enriched with the more volatile component, allowing it to be selectively evaporated, condensed, and collected, thereby separating it from the less volatile component.
Distillation is widely used in both laboratory and industrial applications, such as refining petroleum, isolating fermentation products, and purifying water.
In oil refineries, fractional distillation is employed: crude oil is heated at the base of a tall fractionating column, causing many components to vaporize. As the vapors rise and cool in different zones of the column, they condense and are collected as distinct fractions for various uses (e.g., diesel, kerosene, gasoline).
Simple distillation uses a standard column and is effective when the boiling point difference between the liquids is large.
Vacuum distillation operates under reduced pressure, which lowers the boiling points of all components to avoid decomposition that could occur at higher temperatures.
Chromatography is a separation technique that relies on the differential distribution of components between two phases: a mobile phase and a stationary phase. In this process, the analyte, or substrate, partitions between these two phases based on its relative affinity for each, allowing for effective separation of the mixture’s components.
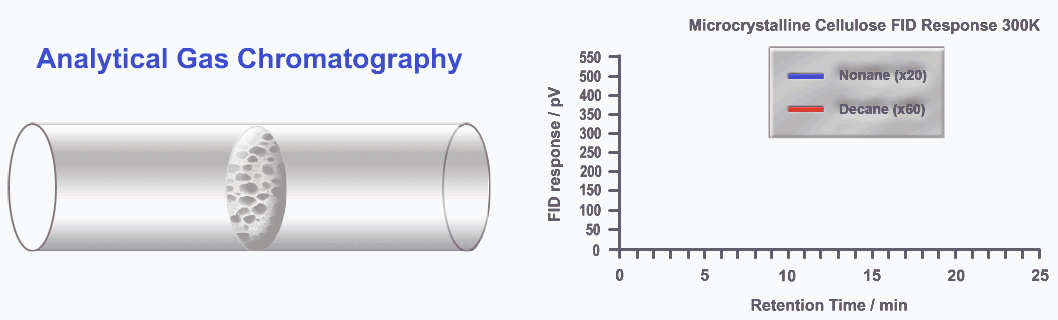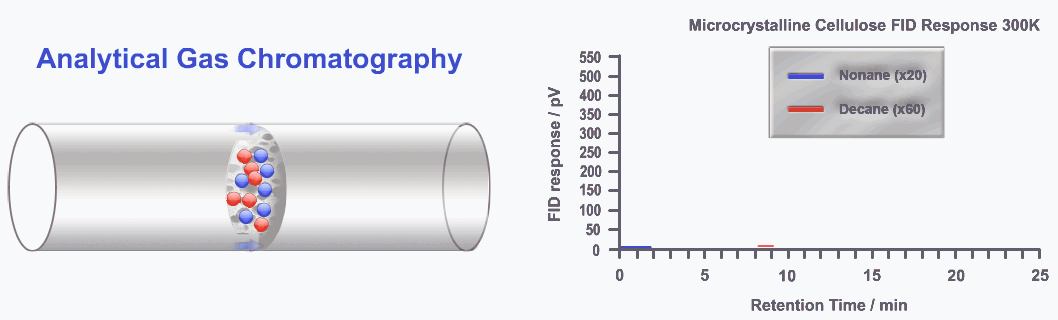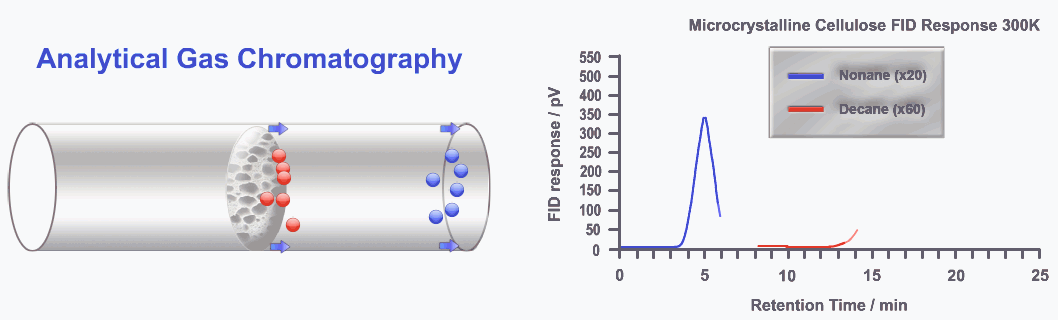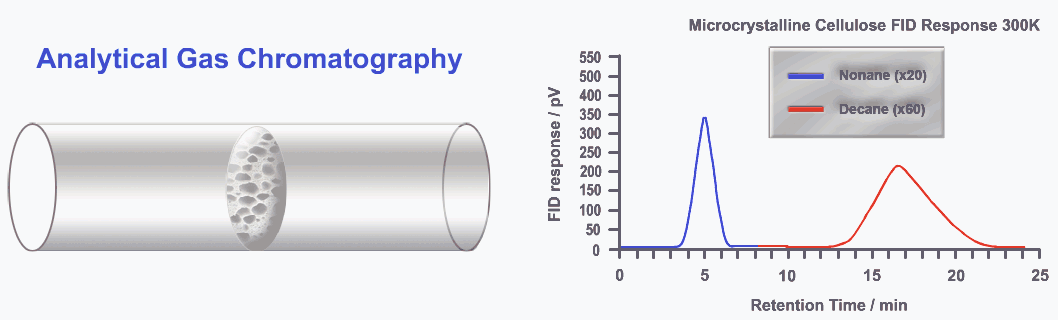
Gas-liquid chromatography (also known as gas chromatography), is ideal when the analyte can be converted into a gas. In this technique, the mobile phase is a gas, while the stationary phase is a liquid coating applied to the inner walls of a column. As the sample moves through the column, its components equilibrate between the gaseous and liquid phases.
Components that have a stronger attraction to the stationary phase move more slowly, so a polar substrate will interact more with a polar stationary phase, and a hydrophobic substrate will favor a hydrophobic stationary phase, thus emerging from the column at different times.
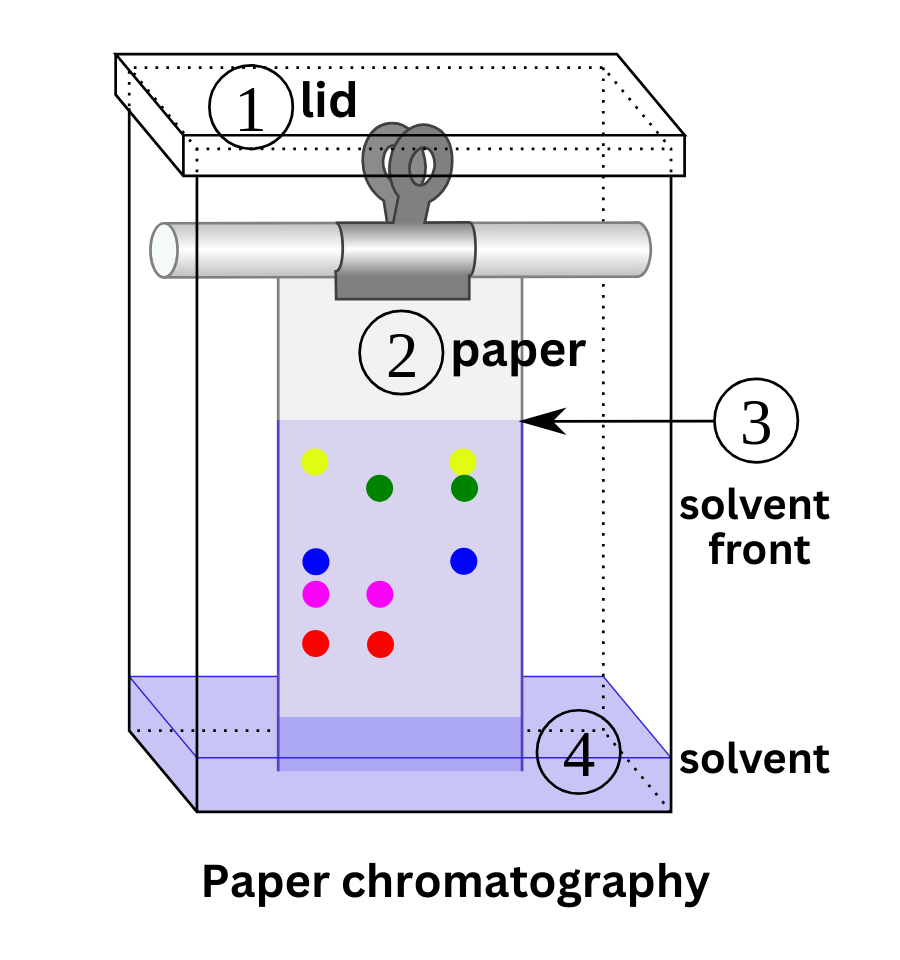
Paper chromatography, which has traditionally been used to separate pigments found in dyes. Here, the solvent serves as the mobile phase, and the paper acts as the stationary phase. As the solvent migrates through the paper, it carries the pigments along with it. Pigments with a higher affinity for the paper remain closer to the original spot, while those more soluble in the solvent travel further.
The separation is quantified by the Rf value, defined as the ratio of the distance traveled by the pigment to the distance traveled by the solvent front. An Rf value of 0 means the pigment has not moved, whereas an Rf value of 1 indicates that it has moved as far as the solvent front.
Thin-layer chromatography, a more advanced variant, operates on the same principles as paper chromatography. In this technique, a plate coated with a specific stationary phase is used instead of paper. The Rf value is calculated in the same way, providing a means to compare the movement of components under controlled conditions.
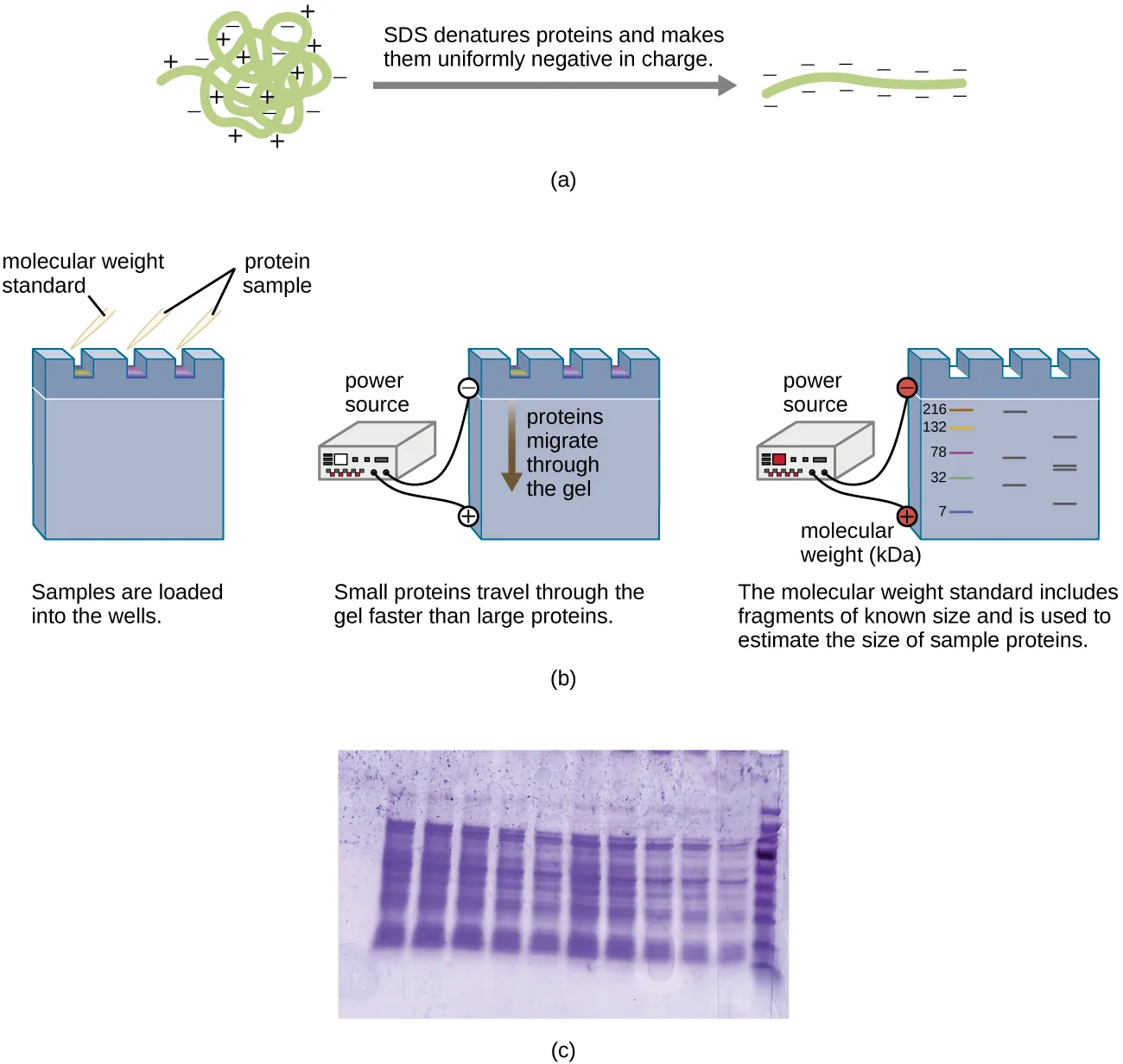
Peptides and proteins can be isolated and purified according to their size or charge, then structurally characterized using several techniques. One prominent method is gel electrophoresis, in which proteins or peptides are loaded into a slot near the negatively charged electrode on a porous matrix (commonly polyacrylamide gel). As an electric field is applied, these molecules migrate toward the positive electrode, with smaller or more compact proteins traveling faster through the pores. To monitor size, a molecular weight standard is typically run alongside the samples for comparison.
Proteins can be analyzed in their native form (native-PAGE) or under denaturing conditions (SDS-PAGE). In native-PAGE, proteins retain their tertiary structure and are separated based on both size and shape; larger or more globular proteins will move more slowly. Under denaturing conditions, the chemical SDS unfolds proteins so that they are primarily separated by size alone. Any disulfide bonds that remain can be further broken using a reducing agent, ensuring that polypeptides are completely unfolded.
Another powerful technique is size-exclusion chromatography, in which molecules in solution pass through a column packed with porous beads of varying pore sizes. Smaller molecules can enter these pores and thus take longer to move through the column, while larger molecules bypass the pores and elute more quickly. As a result, smaller proteins or peptides have longer retention times, while larger ones exit the column sooner.
Lastly, ion exchange chromatography separates proteins by charge using a charged stationary phase. In cation-exchange chromatography, the stationary phase is negatively charged and thus binds positively charged species (cations), while anion-exchange chromatography uses a positively charged stationary phase to capture negatively charged species (anions). Once bound, these proteins can be released (eluted) by passing a solution with a higher ionic strength or by adjusting the pH to alter the protein’s net charge.
Once peptides and proteins are separated, a variety of analytical methods can further identify and characterize them:
Racemic mixtures contain equal proportions of two enantiomers, which are non-superimposable mirror images of each other despite having identical connectivity. Since each enantiomer rotates plane-polarized light in opposite directions but with equal magnitude, a 50:50 mixture results in no net rotation, making the solution optically inactive. When these enantiomers are isolated from one another, the process is known as resolving the racemic mixture.
Sign up for free to take 8 quiz questions on this topic