Ions in solution are charged particles that result when compounds dissolve in water. These ions are of two types: anions (negatively charged) and cations (positively charged).
When these ions are in solution, water molecules surround them in a process called hydration (or solvation). In hydration, the partially negative oxygen atoms of water molecules attract and surround cations, while the partially positive hydrogen atoms surround anions. For example, a free H⁺ ion is not found in aqueous solution; it bonds with a water molecule to form the hydronium ion (H₃O⁺).
Solubility refers to the extent to which a substance dissolves in a solvent and is quantified using various units of concentration. Molarity (M) is defined as moles of solute per liter of solution, while molality (m) is moles of solute per kilogram of solvent. Normality (N) reflects the effective molarity of the reactive species (e.g., ). Concentration can also be expressed as a percentage by mass (x% = x g per 100 g or 100 mL) or in parts per million (ppm) (x mg per kg or per liter).
The solubility product constant () is the equilibrium constant for the dissolution of a sparingly soluble salt. For example, the dissolution of silver chloride is written as:
with an equilibrium expression of:
For a salt like silver sulfate:
the expression is:
A higher value indicates that more of the salt dissolves
Common-ion effect applies Le Chatelier’s principle to solubility equilibria. For example, consider the dissolution of silver chloride:
When a salt containing the common ion (such as sodium chloride, ) is added, the concentration of increases. This shifts the equilibrium to the left, reducing the solubility of . In laboratory separations, the common-ion effect is used to selectively precipitate one component from a mixture by adding a source of an ion that is common to the desired precipitate.
In contrast, complex ion formation can enhance solubility. When a metal ion (M⁺) interacts with a Lewis base (L), it forms a complex ion:
The formation constant, , quantifies this reaction. By forming a complex, the concentration of free metal ions decreases, shifting the solubility equilibrium of a sparingly soluble salt to dissolve more of the solid.
Solubility can also be influenced by pH. For a weak acid () that dissociates as:
Adding a base removes from the solution, driving the equilibrium to the right and increasing the solubility of HA. Conversely, a weak base (B) will dissolve more in an acidic solution because the added converts B to its conjugate acid:
The common-ion effect, complex ion formation, and pH adjustments are powerful tools in controlling solubility and are widely used in laboratory separations to selectively precipitate or dissolve compounds.
Titration is an analytical technique used to determine the concentration of an unknown acid or base by reacting it with a known titrant. A key component in many titrations is the indicator, a compound that behaves like a weak acid or base and is present in such small amounts that it does not affect the overall pH of the solution. The indicator exists in equilibrium:
with its acid dissociation constant given by:
At low pH (high [H⁺]), the indicator is mostly in the H-In form, displaying one color. At high pH (low [H⁺]), it is predominantly in the In⁻ form, exhibiting a different color. This color change signals the endpoint of the titration, which typically involves a neutralization reaction:
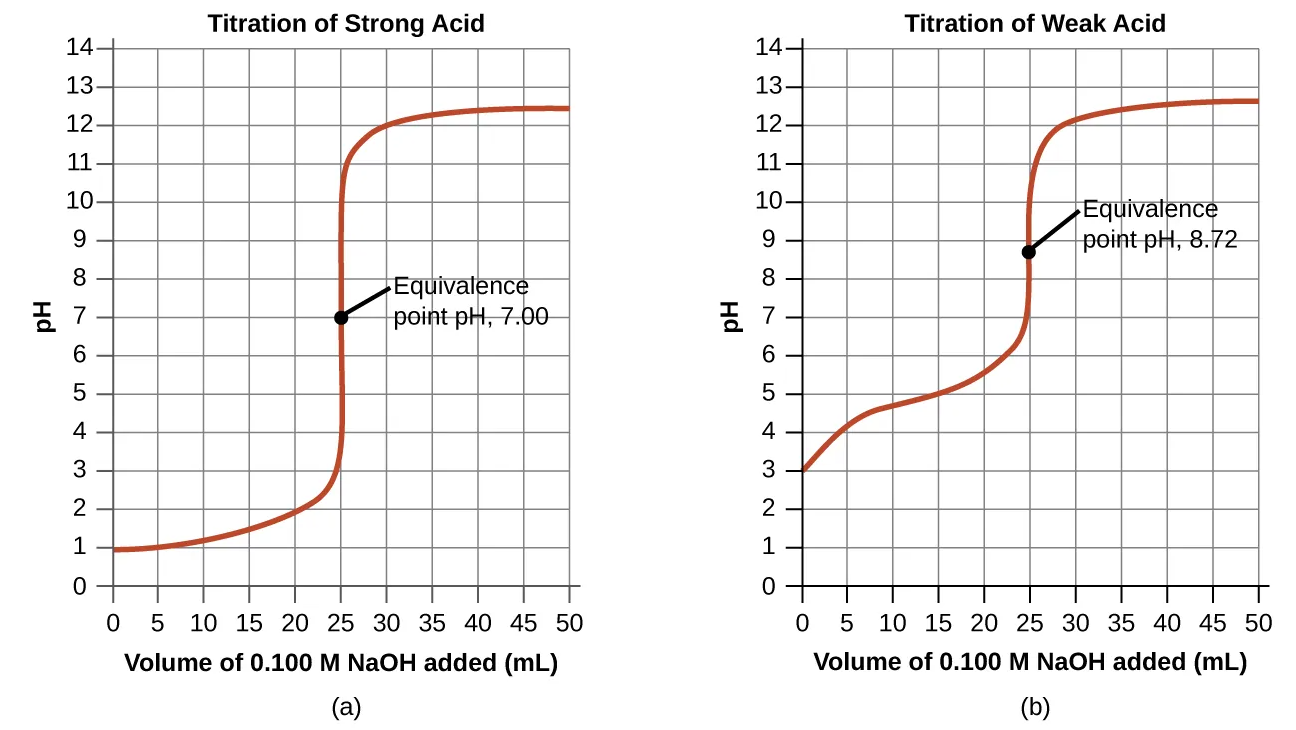
The interpretation of titration curves depends on the nature of the acid or base. For titrations of strong acids with strong bases (or vice versa), the curve shows a sharp transition at the equivalence point. When titrating a weak acid with a strong base or a weak base with a strong acid, a buffer region appears. In this region, the concentrations of the weak acid and its conjugate base (or the weak base and its conjugate acid) are nearly equal, and the pH is approximately equal to the pKa (or 14 minus the pKb). This buffering region usually spans about pKa ± 1.
For weak polyprotic acids, which can donate more than one proton, the titration curve exhibits multiple points of inflection and equivalence points, each corresponding to a different pKa. At each point of inflection, the concentration of the acidic species is equal to that of its conjugate base, allowing for the determination of the individual dissociation constants.
In summary, titration involves careful monitoring of pH changes with the aid of an indicator and uses the principles of neutralization and buffering to accurately determine the concentration of the analyte.
Sign up for free to take 8 quiz questions on this topic