T cell activation
T cell activation: T cell activation begins when a T cell recognizes a foreign peptide presented by an MHC molecule and also receives a co-stimulatory signal from an antigen-presenting cell (APC). Dendritic cells are the most potent activators of T cells.
Adhesion molecules (selectins, integrins, molecules from the immunoglobulin superfamily, mucin-like molecules) and chemokines help T cells home to and transmigrate into lymph nodes, where they can interact with APCs.
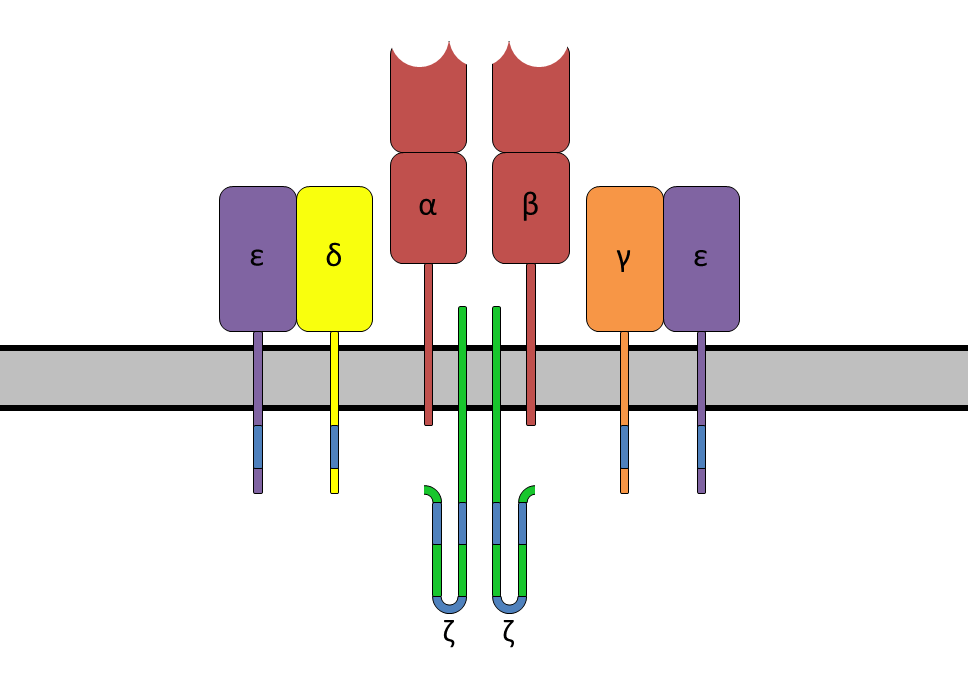
Initially, T cells bind to APCs with low affinity through LFA 1-ICAM 1 interactions. If the TCR (T cell receptor) recognizes the specific peptide antigen presented by the corresponding MHC, LFA 1 undergoes a conformational change that increases its affinity for ICAM 1 and ICAM 2, prolonging contact. At this point:
- Adhesion molecules are bound to each other.
- The TCR is bound to antigen.
- CD4 or CD8 co-receptors are bound to MHC II and MHC I, respectively.
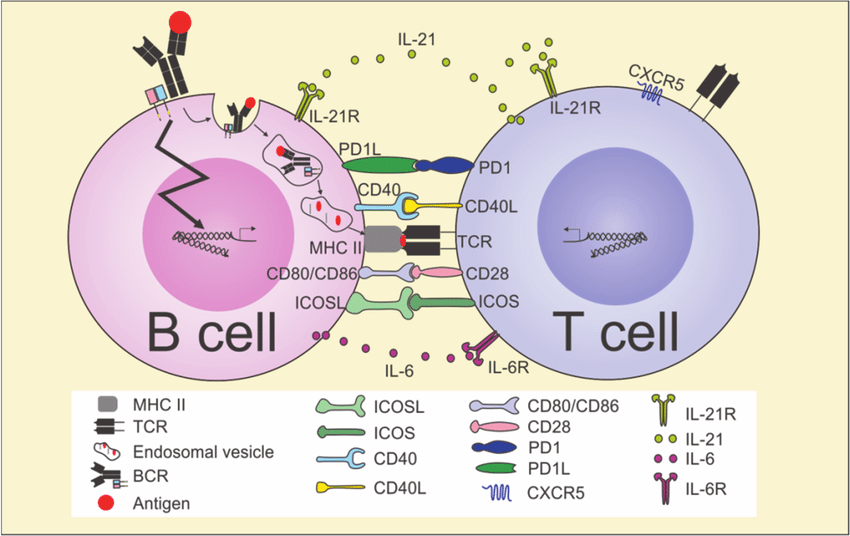
This is followed by a “co-stimulatory” signal provided by interaction of CD 28 on the T cell surface with B7 on the APC. Co-stimulation leads to clonal expansion of naive T cells into effector T cells.
Activated T cells express CD40 ligand, which binds to CD40 on APCs and stimulates increased expression of B7 on the APC. In this way, the T cell and APC reciprocally activate each other.
T cell proliferation is checked by a negative signal induced when CTLA 4 (CD152) on T cells binds B7 (CD80 or 86) on the APC.
In the absence of costimulation, peptide antigen binding to the TCR fails to activate the T cell, leading to a state called anergy. In anergy, the T cell becomes refractory to that antigen even if it is presented later by an APC. This is one mechanism for developing tolerance to self-antigens.
Activated T cells produce IL2 and the alpha chain of the IL2 receptor. When a T cell is activated, signal transmission by CD 3 activates the phospholipase C pathway, which releases intracellular Ca. Ca then activates calcineurin, which activates the genes for IL2 and IL 2 receptor synthesis. IL2 binds to its receptor on the T cell and induces proliferation in an autocrine fashion.
IL2 is a major factor that determines:
- Differentiation and survival of CD4+ T helper subsets and CD4+ T regulatory cells
- Activation of cytotoxic effector lymphocytes
- Formation of memory T cells
The co-stimulatory signal (discussed above) is essential for IL2 production. Anergic T cells cannot produce IL2.
Activated T cells differentiate into effector T cells that function as CD4 or CD8 T cells. CD4 T cells also indirectly enhance activation of CD8 T cells by increasing expression of co-stimulatory molecules on APCs. Effector T cells do not need co-stimulatory signals for their function. They express the integrin VLA 4, which allows them to bind to vascular endothelium.
An activated CD4 T cell can differentiate into either Th1 or Th2 cells.
Comparison between Th1 and Th2 cells
| Th1 cells | Th2 cells |
| Induces cell mediated immunity | Induces humoral immunity |
| IL12 and Gamma interferon favor Th1 series | IL4,6 and 10 favor Th2 series |
| Activates macrophages | Activates B cells |
| Produce gamma interferon, IL2, TNF beta | Produce IL 4,5,6,9,10,13 |
| Seen in viral, fungal, intracellular bacterial infections, delayed hypersensitivity | Seen in allergies, parasitic infestations, bacterial infections |
B cell antigen receptor and coreceptors: The B cell antigen receptor is made of an antibody molecule that recognizes antigen, plus transmembrane regions (carboxy terminals of the heavy chains) involved in signal transduction. The cell surface immunoglobulin (Ig) that binds antigen has the same specificity as the antibodies secreted by that B cell.
In addition, one Ig alpha and one Ig beta chain are associated with the surface Ig. The Ig alpha and Ig beta chains extend into the cytoplasm and associate with ITAM motifs. When antigen binds, the tyrosines in these ITAMs are phosphorylated by receptor-associated Src-family tyrosine kinases (Blk, Fyn, or Lyn). This is followed by activation of Syk kinase, which initiates a signal cascade involving BLNK, CD19, Tec kinases (like Bruton’s tyrosine kinase), and Ras, as well as phospholipase C (Ca++, IP3, and DAG) second messenger systems.
The end result is activation of transcription factors (Fos, Jun, NFAT, and NFkB), causing cell proliferation and differentiation. MAP kinases are activated through pathways such as Ras, with a resulting signal cascade involving Raf, Mek, and Erk, leading to increased transcription.
The B cell coreceptor is a complex of cell surface molecules CD19, CD 21 (or complement receptor CR 2), and CD 81. Antigen or complement C3d can cross-link the B cell receptor with the coreceptor. This results in phosphorylation of the cytoplasmic tail of CD 19 by tyrosine kinases and a signal transduction cascade involving phosphatidylinositol (PI) 3 kinase. Hence, the coreceptor exponentially increases signalling.
B cell activation: Naive (unstimulated) B cells can be activated either by helper T cells or directly by microbial antigens.
- The first signal for activation is delivered by antigen binding to the B cell receptor.
- The antigen is then internalized by the B cell, processed intracellularly, and presented with MHC II on the B cell surface.
- The presented antigen is recognized by a CD4 T cell, which then delivers the second activating signal.
The B cell coreceptor enhances signaling by 100 to 10,000 fold.
Most antigens activate B cells with T cell help and are called “T cell dependent” antigens. Some antigens (such as bacterial capsular polysaccharide, DNA, RNA, and lipids) can activate B cells directly and are called “T cell independent” or TI antigens.
Following antigen recognition, the CD40 ligand on the T cell binds to CD 40 on the B cell and secretes IL4, which stimulates and initiates proliferation of B cells. Eventually, cytokines IL5 and IL6 are also secreted by the T cell, which help drive differentiation of B cells into antibody-secreting plasma cells.
Activated B cells form germinal centers in lymphoid follicles. Cytokines induce isotype switching, which determines the predominant antibody type. IgM is the initial antibody produced.
Relation between cytokines and antibody response
| Cytokine | Antibody produced |
| IL4 | IgG1 and IgE |
| IL5 | IgA |
| Gamma interferon | IgG3 and IgG2 |
| TGF beta | IgG2 and IgA |