
The periodic table organizes elements into groups based on their electronic structure and similar physical and chemical properties. For instance, alkali metals are characterized by having a single valence electron, which gives them a low ionization energy and makes them highly reactive. They tend to lose that electron to achieve a stable, empty valence shell, forming compounds predominantly in the +1 oxidation state. Their reactivity increases down the group due to larger atomic radii, and they typically react with oxygen to form oxides, with water to yield hydroxides while releasing hydrogen, and with acids to form salts with hydrogen gas as a byproduct.
Similarly, alkaline earth metals have two valence electrons and also exhibit relatively low ionization energies, though they are generally slightly less reactive than alkali metals. They lose both electrons to attain a stable electron configuration, commonly resulting in a oxidation state. As with alkali metals, their reactivity increases with an increase in atomic radii, and they too react with oxygen, water, and acids in predictable ways. This systematic arrangement of elements allows us to understand and predict the behavior of different groups within the periodic table.
The halogens are elements that possess 7 valence electrons (2 in the s subshell and 5 in the p subshell), giving them a high electron affinity and making them very reactive. They tend to gain one electron to achieve a complete valence shell, so they typically exist in the -1 oxidation state. Their reactivity increases as you move up the group due to decreasing atomic radii, and they readily react with alkali and alkaline earth metals to form salts.
In contrast, the noble gases have a full valence shell of 8 electrons, which results in a high ionization energy and low electron affinity. This stable electron configuration renders noble gases virtually non-reactive, and they are generally found in the 0 oxidation state.
Transition metals are characterized by their high conductivity, which is due to loosely bound d electrons that can move freely. In chemical complexes, when ligands bind to these metals, the formerly degenerate d orbitals split into nondegenerate energy levels. This splitting allows electrons to transition between different d orbitals, producing the vivid colors seen in many transition metal compounds. These metals also exhibit varied oxidation states, typically positive, which contributes to their rich chemistry.
In contrast, representative elements from the s and p blocks lack free-moving d electrons, and their valence shells fill in a predictable manner from 1 to 8 electrons as indicated by standard group nomenclature (IA, IIA, IIIA, etc.).
Metals are positioned to the left of the metalloids on the periodic table, while non-metals are found to the right. Chemically, metals tend to lose electrons to achieve a positive oxidation state, making them effective reducing agents. They exhibit low electronegativity, which results in a partially positive character in covalent bonds with non-metals and leads them to form basic oxides. Physically, metals are excellent conductors of heat and electricity, are malleable and ductile, display a characteristic luster, and are typically solid at room temperature (except mercury).
In contrast, non-metals tend to gain electrons, resulting in a negative oxidation state and making them strong oxidizing agents due to their higher electronegativity. They form acidic oxides. Physically, non-metals can be solid, liquid, or gas at room temperature; when solid, they are usually brittle and lack the metallic luster found in metals.
The oxygen group (or chalcogens) occupies a single column on the periodic table that begins with oxygen. Within this group, oxygen and sulfur are both non-metals and exhibit similar chemical reactivity, making sulfur a common substitute for oxygen in certain reactions. As you move down the group, the elements display a gradual change in properties: selenium remains a non-metal, tellurium behaves as a metalloid, and polonium exhibits metallic characteristics or is considered a metalloid. This trend reflects the broader periodic arrangement where metals are typically found to the left of metalloids, and the variations in atomic structure influence their reactivity and physical properties.
The electronic structure of atoms varies across different groups in the periodic table. Representative elements, located in the s block and p block, lack free-flowing outer d electrons. Their valence shell fills progressively from one electron on the far left to eight electrons on the far right, and these elements are categorized using standard nomenclature (IA, IIA, IIIA, etc.). For example, the noble gases have a complete valence shell of eight electrons, which results in high ionization energy and low electron affinity, rendering them chemically inert and typically found in the 0 oxidation state.
In contrast, transition metals possess loosely bound outer d electrons that grant them high conductivity and versatile chemical behavior. When transition metals form complexes, the presence of ligands causes their d orbitals to become nondegenerate, meaning they split into different energy levels. Electron transitions between these split d orbitals are responsible for the vivid colors characteristic of many transition metal compounds, and these metals often exhibit varied positive oxidation states due to the flexible participation of their d electrons in bonding.
Valence electrons are the electrons in an atom’s outermost shell that primarily determine its chemical behavior. In representative elements, the number of valence electrons increases from one to eight as you move from left to right across the periodic table. This pattern, however, does not apply to transition metals, where additional electrons in inner d orbitals influence chemical properties.
The first ionization energy is the energy required to remove the first valence electron from an atom, while the second ionization energy is the energy needed to remove a second electron after the first has been removed. These energies are influenced by the atom’s electronic structure.
Noble gases exhibit the highest ionization energies due to their complete valence shells, whereas alkali metals have the lowest, as they contain only one valence electron that is easily removed. Local maxima in ionization energy are observed for elements with filled or half-filled subshells, which confer extra stability. Importantly, the second ionization energy is always significantly higher than the first, especially in alkali metals, where the first electron is weakly held but the remaining electrons are much more strongly bound, and hydrogen, where there is no second electron available after the first electron is removed. In alkaline earth metals, both the first and second ionization energies are relatively low, reflecting their electronic configurations and the energy required to remove electrons.
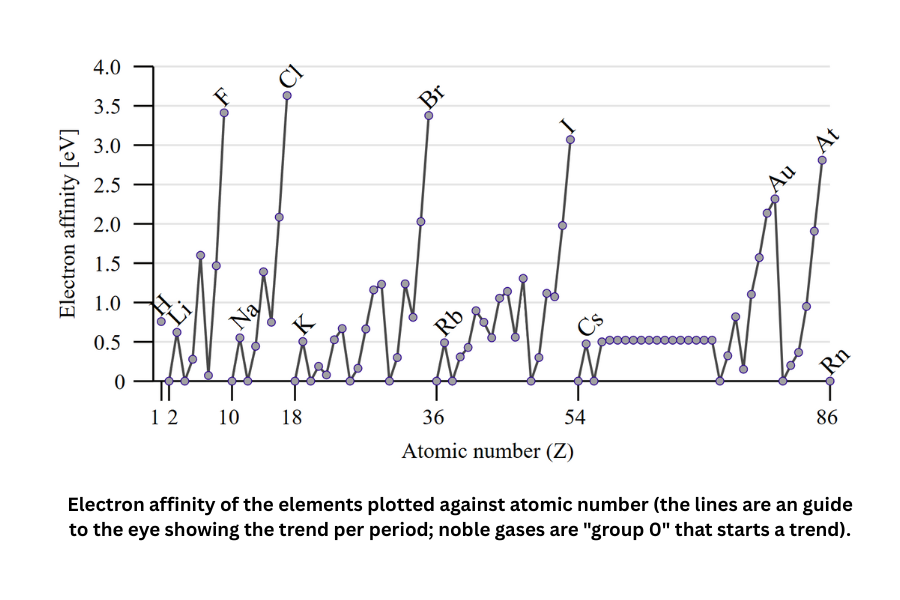
Electron affinity is the energy released when an atom gains an electron, reflecting how easily it can accept an electron. This property generally decreases as you move down a group due to the increase in atomic radii, which weakens the attraction between the nucleus and the added electron. In contrast, as you traverse a period from left to right, electron affinity tends to increase because the atomic radius decreases and the effective nuclear charge strengthens the pull on the electron.
The highest electron affinities are observed in the halogens, while the noble gases exhibit the lowest. Additionally, local minima in electron affinity occur for atoms with filled subshells or half-filled p subshells, which are particularly stable and less inclined to release energy upon electron gain.
Electronegativity is a measure of an atom’s ability to attract electrons within a covalent bond. Atoms with higher electronegativity tend to retain a larger share of electrons, acquiring a partial negative charge, while those with lower electronegativity (or that are more electropositive) receive less electron density and develop a partial positive charge. Electronegativity generally increases as you move toward the top right of the periodic table, with fluorine being the most electronegative element. Elements surrounding fluorine, such as nitrogen, oxygen, chlorine, and bromine, are also highly electronegative, which is a common characteristic among halogens.
Non-metals typically have higher electronegativity compared to metals, and some noble gases like krypton and xenon can exhibit significant electronegativity when they participate in bonding. When the difference in electronegativity between two atoms becomes too large, the bond formed is not covalent but rather ionic, resulting from the complete transfer of electrons from the electropositive atom to the electronegative atom.
Electron shells are the regions around the nucleus defined by the principal quantum number ().
The size of an ion is determined by the number of electrons and the effective nuclear charge. When an atom loses electrons to form a cation, the reduction in electron-electron repulsion and the unchanged nuclear charge cause the ion to shrink compared to the neutral atom. Conversely, when an atom gains electrons to form an anion, the increased electron repulsion makes the ion larger than its parent atom.
Sign up for free to take 7 quiz questions on this topic