Molecular structure and absorption spectra provide insights into a molecule’s composition through different regions of electromagnetic radiation.
Infrared light absorption is due to intramolecular vibrations and rotations, with specific functional groups exhibiting characteristic absorptions that form a unique fingerprint region. Electronic transitions can also produce infrared radiation. Its frequencies extend just below red in the visible spectrum, hence the term “infrared” (below red). At these higher frequencies, only atoms and molecules can vibrate rapidly enough to emit or absorb such radiation. Water molecules are especially active in the infrared, which is why skin has an emissivity of about 0.97 in this region; night-vision scopes detect the infrared glow from warm objects and convert it to visible light.
The Sun behaves much like a blackbody at about 6000 K:
On average, half of this solar energy is absorbed by Earth. The Earth radiates primarily in the infrared, with much of that absorbed by and in the atmosphere, then re-radiated—known as the greenhouse effect—keeping Earth’s surface about C warmer than it would otherwise be.
Visible light absorbed by a molecule gives rise to its complementary color—for example, carotene absorbs blue light, making it appear orange—and structural changes, as seen in indicators, can shift these absorptions. Indicators are substances that “change color” when exposed to a certain pH or metal ions, or flouresce when exposed to certain bands of visible light.
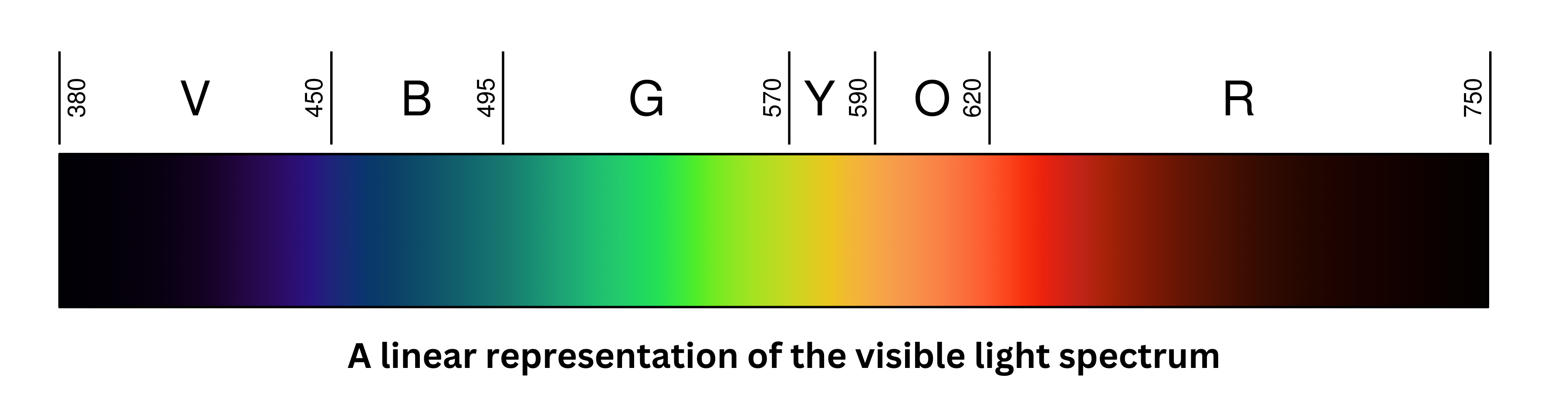
Visible light usually spans 400 nm to 750 nm in wavelength, with red having the lowest frequencies and longest wavelengths, and violet having the highest frequencies and shortest wavelengths. Although the retina can detect some ultraviolet, these rays are typically blocked by the eye’s cornea and lens.
The Sun emits more intensely at red than violet, giving it a yellowish cast. Living organisms, such as plants, harness only a portion of the visible spectrum (e.g., in photosynthesis) for essential biological processes.
Ultraviolet (UV) radiation lies just above violet in the electromagnetic spectrum, spanning from 400 nm down to about 10 nm. Ultraviolet absorption occurs when -electrons and non-bonding electrons in a molecule transition to higher energy levels. In conjugated systems, where electrons are delocalized across multiple bonds, these transitions happen at longer wavelengths, since the extended delocalization lowers the energy gap, shifting the absorption into the UV–visible range.
Solar UV is split into UV-A (320–400 nm), UV-B (290–320 nm), and UV-C (220–290 nm). Most UV-B and all UV-C are absorbed by ozone in the upper atmosphere, leaving UV-A as the primary form reaching the Earth’s surface.
Despite the small fraction of UV-B that arrives at ground level, it is the primary cause of skin cancer and sunburn, damaging DNA by distorting its helix. UV can also break down collagen fibers, hastening the aging of skin and producing wrinkles. The tanning response helps protect living cells by placing pigments in inert skin layers.
Beyond its harmful effects, UV is used to treat infantile jaundice and some skin ailments, sterilize workspaces, and identify substances through fluorescence. Special black lights emit UV to make some objects glow visibly, while UV microscopes enhance resolution beyond what is possible with visible light.
The UV dose (expressed in ) is found by taking the power density () and multiplying it by the duration of exposure (seconds).
Example. A black light has a power density of . If a surface is exposed for 6 minutes (360 seconds), what is the UV dose in ?
Multiplying power density () by time () gives:
Nuclear magnetic resonance (NMR) is an absorption spectroscopy technique that examines certain atomic nuclei, such as hydrogen (protons), interacting with an externally applied magnetic field and absorbing energy in the radiofrequency range and revealing detailed information through spin-spin splitting patterns. These nuclei possess a property called spin, making them behave like tiny magnets that “resonate” or flip between energy levels at specific frequencies.
Magnetic resonance imaging (MRI) is based on NMR and produces detailed two- or three-dimensional images of the body without using x-rays. In a laboratory setting, NMR is used to analyze molecular structure by examining the positions, intensities, and splitting patterns of peaks in the NMR spectrum, thus providing insights into both molecular structure and dynamics.
Sign up for free to take 7 quiz questions on this topic