Light is a form of electromagnetic radiation that behaves as a wave, exhibiting phenomena such as interference. A classic example is the Young’s double-slit experiment, where light passes through two parallel slits. For interference to occur, the light from both slits must be coherent, meaning it maintains a constant phase relationship, and monochromatic, having only one wavelength.
The resulting pattern is described by the equation
where is the distance between the slits, is the angle of the fringe relative to the center, is the order of the fringe, and is the wavelength.
Diffraction is another wave-like behavior of light when it interacts with various obstacles and media. In thin films, light reflects off both the outer and inner surfaces, causing the reflected waves to interfere with each other; this interference produces colorful patterns, as seen in an oil film on water.
When light encounters a small opening or obstacle, it does not simply form a clear image of that object; instead, it produces a pattern of interference due to diffraction. For instance, when light shines through a pinhole, a series of concentric bright and dark circles appears on the screen, with a prominent bright spot at the center.
Similarly, when light passes the edge of an opaque boundary, it does not create a sharply defined shadow; instead, fringes of alternating brightness form along the edge. In another example, light passing by a small object like a penny creates a shadow that is not completely dark, but rather shows a bright central spot surrounded by rings of light and dark, demonstrating the wave nature of light.
In X-ray diffraction, high-energy X-rays are directed at a crystal, where they diffract off the regularly spaced atoms. The resulting interference pattern, which consists of a series of spots or rings, provides detailed information about the molecular structure of the crystal.
Polarization of light describes the orientation of its electric field oscillations. In unpolarized light, the electric field vibrates in multiple directions, whereas in polarized light, it is confined to a single plane.
This characteristic can be observed and utilized in several ways:
The Doppler effect describes how the frequency of light changes when there is relative motion between the light source and the observer. When the source and observer move apart, the observed frequency decreases, resulting in a red shift, which is why astronomical objects moving away from us often appear redder than they are. Conversely, when they move toward each other, the observed frequency increases, producing a blue shift, causing the light to appear bluer. The equations used for these shifts are analogous to those for sound waves, except that the speed of light replaces the speed of sound.
…where:
| Doppler shift | Stationary observer | Observer moving towards source | Observer moving away from source |
|---|---|---|---|
| Stationary source | |||
| Source moving towards observer | |||
| Source moving away from observer |
The photon model of light treats electromagnetic radiation as packets of energy called photons, each possessing energy proportional to its frequency. This relationship is given by , where is Planck’s constant and is the frequency. Because wavelength () and frequency are connected by the speed of light (), another useful formula for photon energy is .
The electromagnetic spectrum spans a wide range of radiation types, distinguished by their energy and wavelength:
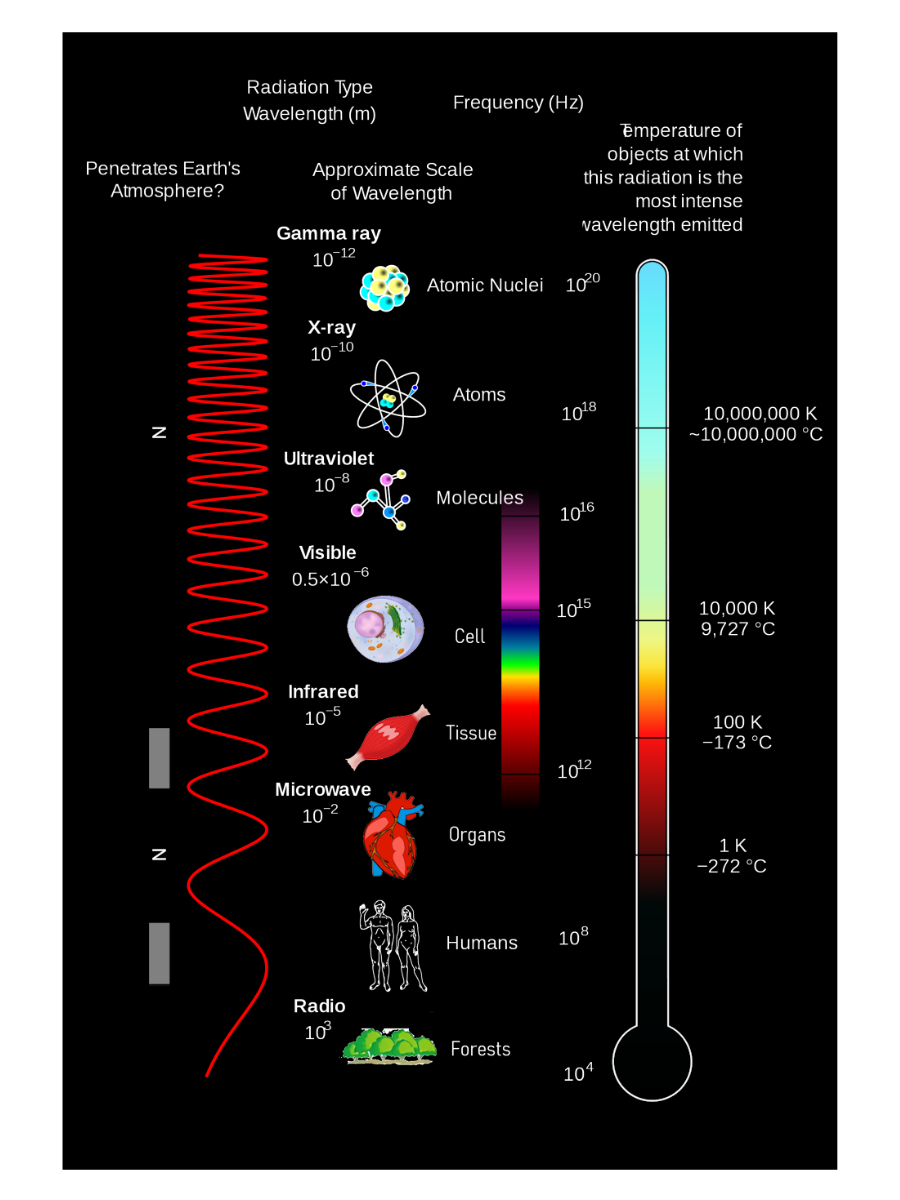
Electromagnetic radiation travels at a maximum speed of (approximately ) in a vacuum. When it passes through a medium, its velocity decreases according to the relationship , where is the index of refraction, is the radiation’s speed in that medium, and is the speed of light in a vacuum. As an electromagnetic wave, it consists of oscillating electric and magnetic fields at right angles to each other and to the direction of propagation.
The electromagnetic spectrum ranges from low-frequency, long-wavelength radiation to high-frequency, short-wavelength radiation. At the lower-frequency end are radio waves, which cause electronic oscillations in antennas. Slightly higher in frequency, microwaves induce molecular rotation, while infrared waves lead to molecular vibration.
Visible light can excite electrons within atoms, spanning approximately 400 nm (violet) to 700 nm (red).
Ultraviolet waves carry enough energy to break bonds or eject electrons—often classified as ionizing radiation. X-rays, still more energetic, also ionize matter and can trigger the photoelectric effect.
The highest frequencies belong to gamma rays, which have even greater energies and penetrating power than X-rays.
The visual spectrum spans a range of wavelengths and frequencies corresponding to different colors. Blue light, with its shortest wavelength and highest frequency, carries the most energy per photon (), where is Planck’s constant and ν is frequency), while red light, with its longest wavelength and lowest frequency, has the least energy.
A laser—an acronym for Light Amplification by Stimulated Emission of Radiation—produces light through stimulated emission rather than the spontaneous emission typical of ordinary light. Within the lasing medium, light is repeatedly amplified by reflecting it back and forth, resulting in a coherent and highly focused beam.
Sign up for free to take 11 quiz questions on this topic