The absolute temperature scale, measured in Kelvin , places its zero point at absolute zero, the lowest possible temperature where no further heat energy can be extracted from a substance. This scale aligns directly with the Celsius scale by using the relation . As a result, water freezes at () and boils at .
Though the Fahrenheit () scale remains widely used in some regions (where ), the Kelvin scale is fundamental in scientific contexts because it begins at the theoretical point of zero thermal energy.
| Description | Celsius () | Kelvin () | Fahrenheit () |
| Surface of the sun | 5600 | 5900 | 10100 |
| Boiling point - water | 100 | 373 | 212 |
| Body temperature | 37 | 310.2 | 98.6 |
| Room temperature | 20 | 293 | 68 |
| Cool day | 10 | 283 | 50 |
| Freezing point - water | 0 | 273 | 32 |
Pressure is defined as the force exerted per unit area, expressed by the equation
where represents the applied force and is the area over which this force acts.
At sea level, the atmospheric pressure is about , equivalent to , and it decreases with increasing elevation.
A mercury barometer measures this pressure by allowing the atmosphere to push a column of mercury upward; one end of the barometer is open to the air while the other is sealed to form a vacuum.
Molar volume at 0 degrees Celsius and is 22.4 . This means that ideal gases occupy 22.4 liters per mole of molecules. Remember, it’s 22.4 liters per mole, not the reverse.
A mole consists of approximately particles, which is an enormous number of molecules that take up a considerable volume. For instance, consider that air is mostly composed of nitrogen; in its diatomic form (), nitrogen has a molar mass of about 28 grams. Because air is very light, a full mole of it occupies 22.4 liters at standard conditions.
An ideal gas is defined by the kinetic molecular theory, which models the gas as a collection of point particles that move randomly and collide elastically with each other and the container walls.
In an ideal gas, the molecular volume is negligible and there are no intermolecular forces. This ideal behavior is observed under conditions of low pressure and high temperature, where molecules are sufficiently separated; however, at high pressures and low temperatures, the proximity of molecules leads to significant intermolecular interactions and volume effects, eventually causing the gas to condense.
This law underlies the combined gas law, which shows that when and are constant, the ratio remains constant. From this relationship, Boyle’s law ( at constant ) and Charles’s law ( at constant ) can be derived, as well as Avogadro’s law, which states that equal volumes of gas at the same temperature and pressure contain equal numbers of moles.
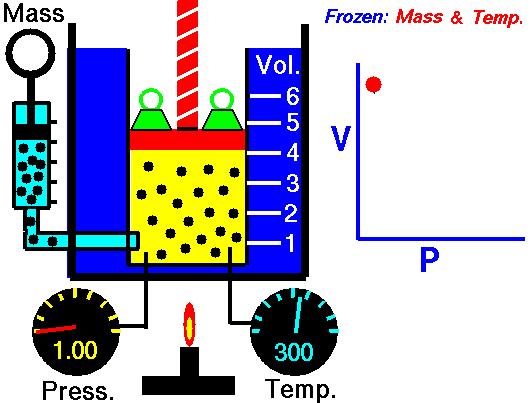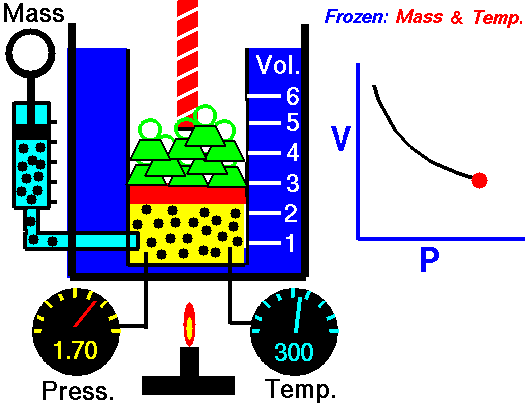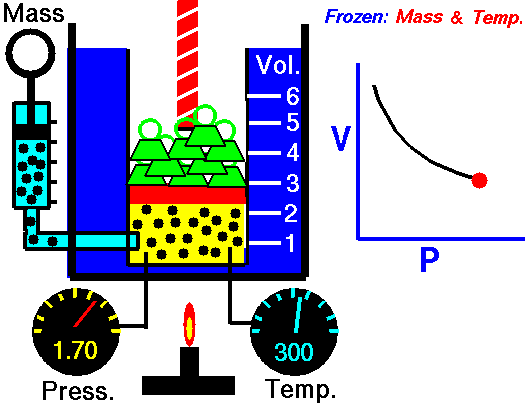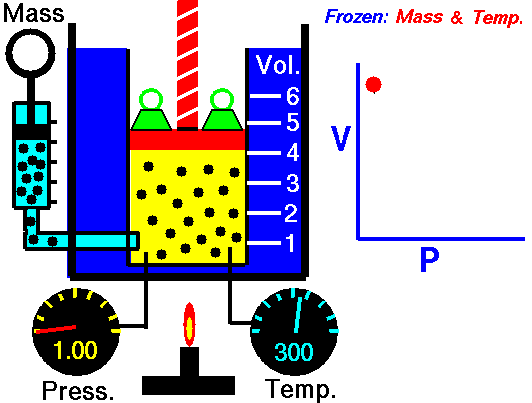
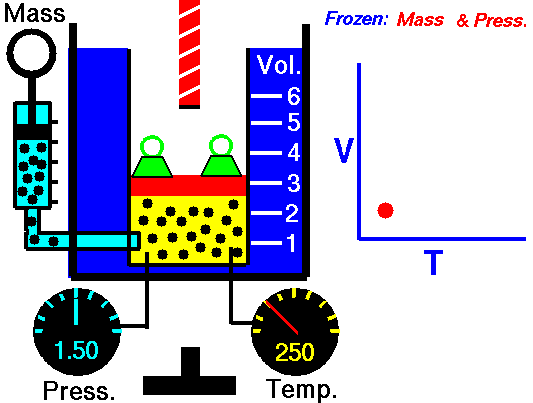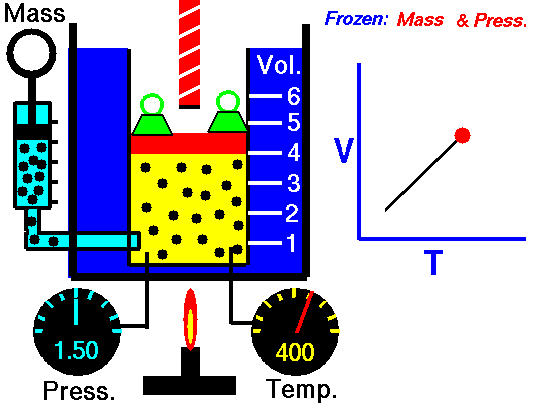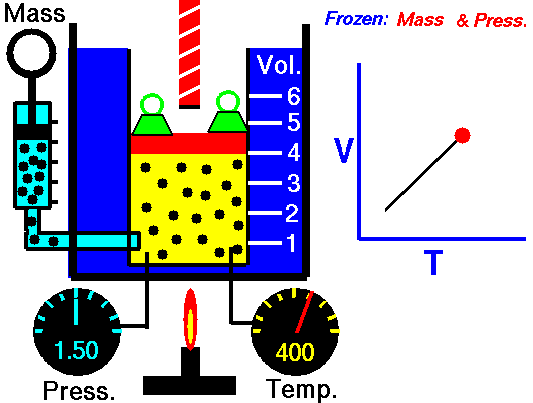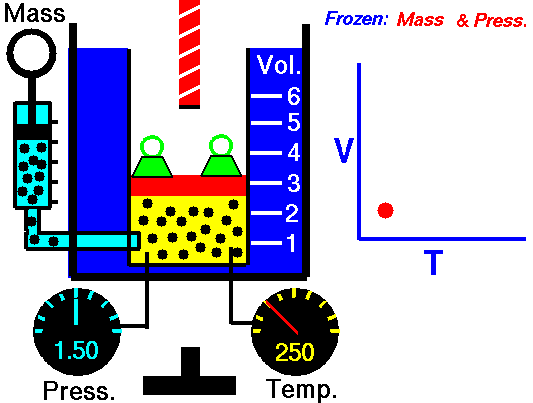
The kinetic molecular theory explains the behavior of gases and underpins the ideal gas laws. It assumes that gas molecules are in constant, random molecular motion, experience no intermolecular forces, and have negligible molecular volume. Additionally, all collisions are perfectly elastic collisions, ensuring that the total kinetic energy remains constant during interactions.
In this model, pressure arises from the molecules colliding with the container walls, and because these collisions occur randomly, the pressure is evenly distributed throughout the container. Temperature is a measure of the average kinetic energy of the gas molecules, so
The heat capacity at constant volume is the amount of heat required to raise a system’s temperature by one degree while keeping its volume fixed, so that no work is done by expansion.
In contrast, the heat capacity at constant pressure is the heat needed to increase the temperature by one degree while the pressure remains constant; in this case, some energy is used for expansion work, making it higher than the constant-volume heat capacity. The difference between these two is accounted for by the work done against the surroundings.
Boltzmann’s constant is a fundamental value that connects the average kinetic energy of individual particles with the macroscopic temperature of a system, serving as a bridge between microscopic behavior and bulk thermal properties.
Diffusion is the process in which molecules move randomly from regions of higher concentration to lower concentration, effectively traveling down their concentration gradient.
In contrast, effusion occurs when gas molecules escape through a very small opening in a container, again driven by their random motion.
In an ideal gas, molecules are assumed to have negligible volume and no interactions, a good approximation at low pressure and high temperature when they are far apart.
However, at higher pressures or lower temperatures, molecules are closer together and begin to experience intermolecular attractions, which pull them toward each other and effectively reduce the pressure. When they are forced into extremely close proximity, their finite sizes result in steric repulsion, which pushes them apart and increases the pressure.
These deviations from ideal behavior are quantitatively described by the Van der Waals equation, which introduces two constants: accounts for attractive forces (lowering the pressure) and represents the finite molecular volume (raising the pressure).
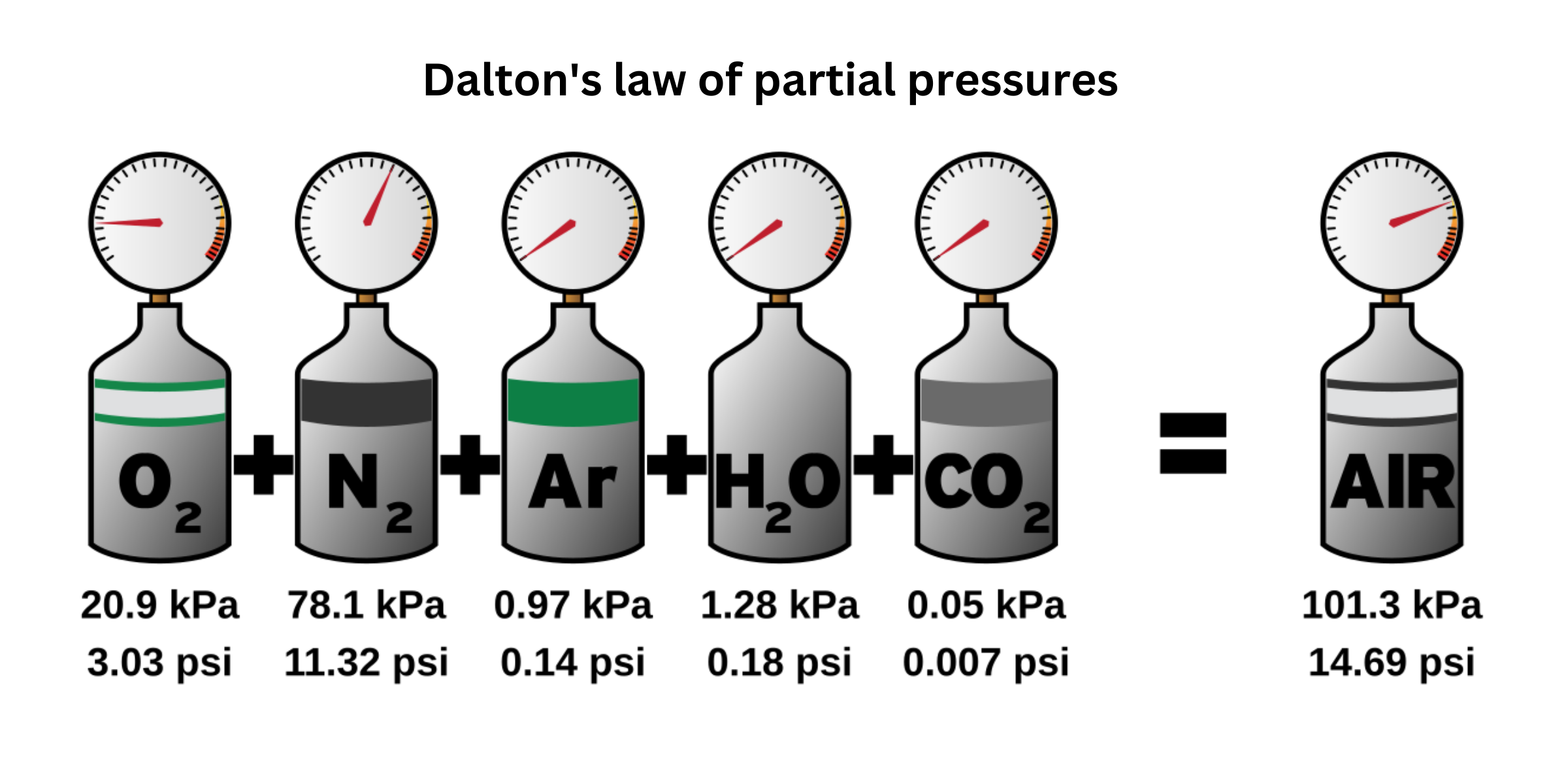
Partial pressure is the pressure exerted by an individual gas within a mixture, and the total pressure is the sum of all these partial pressures.
The proportion of a specific gas in the mixture is given by its mole fraction, defined as the number of moles of that gas divided by the total number of moles in the mixture.
Sign up for free to take 9 quiz questions on this topic