Metabolism encompasses the full range of chemical processes in living organisms, divided into two complementary aspects: catabolism (breaking down molecules to release energy) and anabolism (using energy to build and store complex molecules).
In aerobic metabolism, oxygen is required to completely oxidize a source of fuel—commonly glucose—into carbon dioxide and water while capturing released energy in the form of ATP. Under these conditions, one molecule of glucose typically yields around 30 ATP. The overall reaction is often summarized as:
Glycolysis is the central metabolic pathway in which glucose is broken down into pyruvate, yielding small amounts of ATP and NADH.
Under aerobic conditions, pyruvate enters the mitochondria to fuel further energy production via the citric acid cycle and oxidative phosphorylation.
Steps in glycolysis:
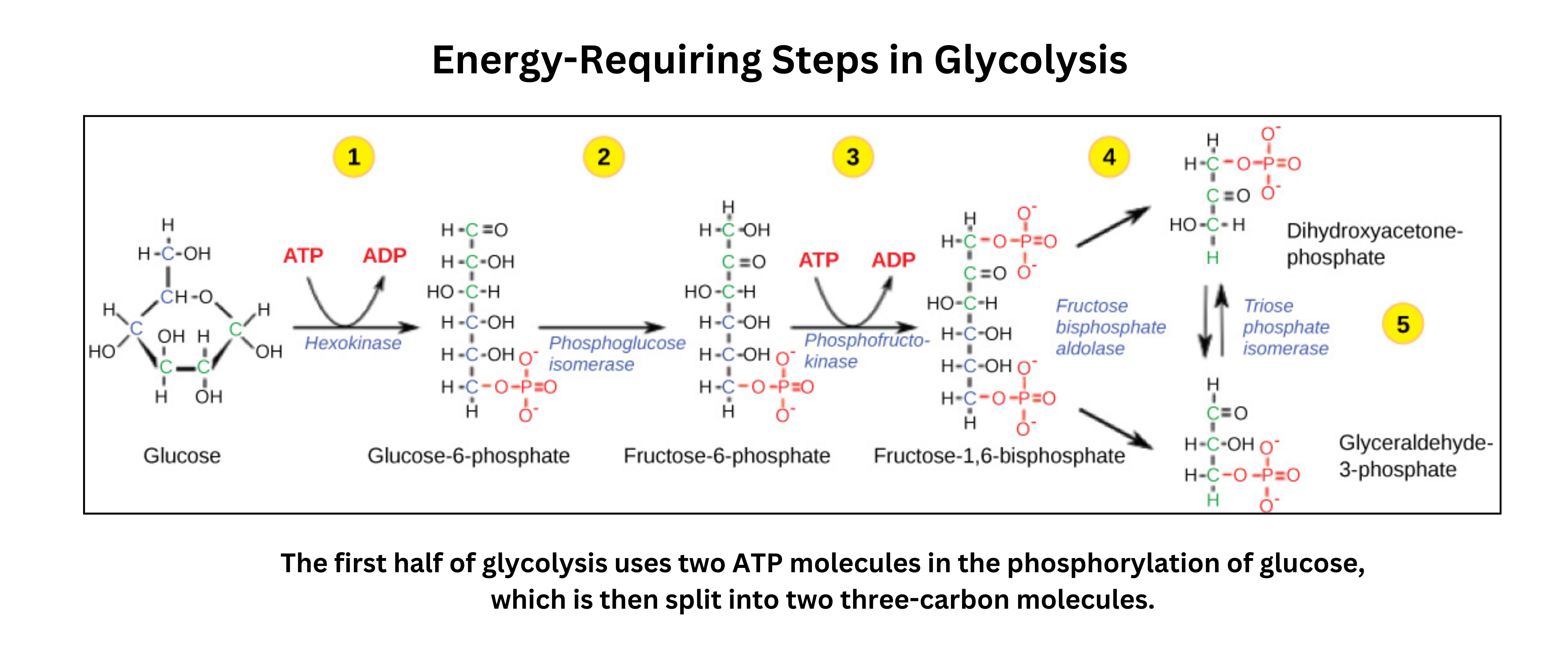
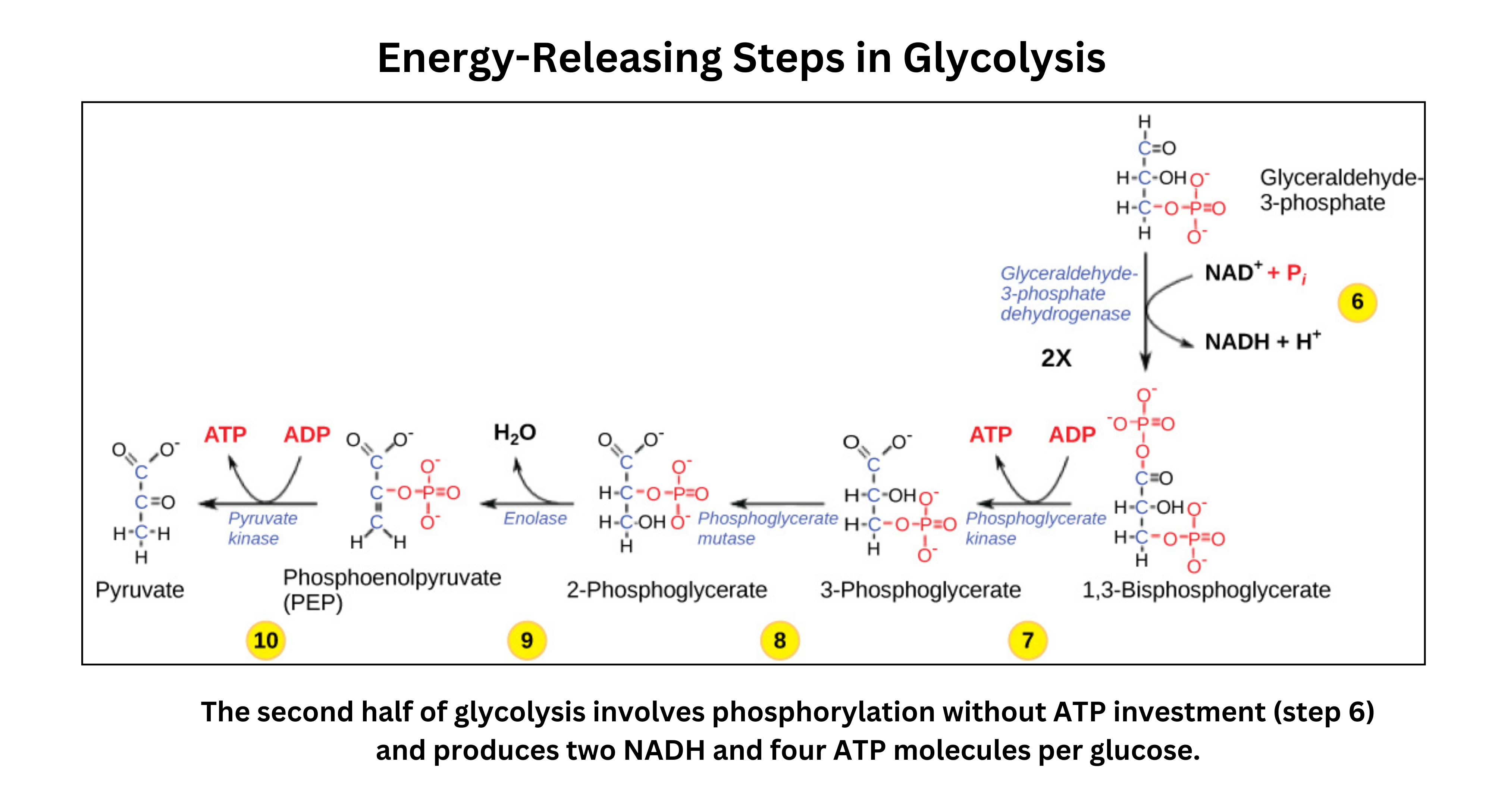
However, glycolysis also serves as the endpoint for anaerobic metabolism, or fermentation. Fermentation starts with partial oxidation of glucose to pyruvate, with 2 net ATP produced per glucose. Then, pyruvate is converted into lactate (in animals), called lactic acid fermentation, or ethanol and carbon dioxide (in yeast), called alcohol fermentation, to regenerate NAD⁺.
Carbohydrates stored as glycogen in animals or starch in plants can be rapidly mobilized via feeder pathways, providing additional glucose for glycolysis.
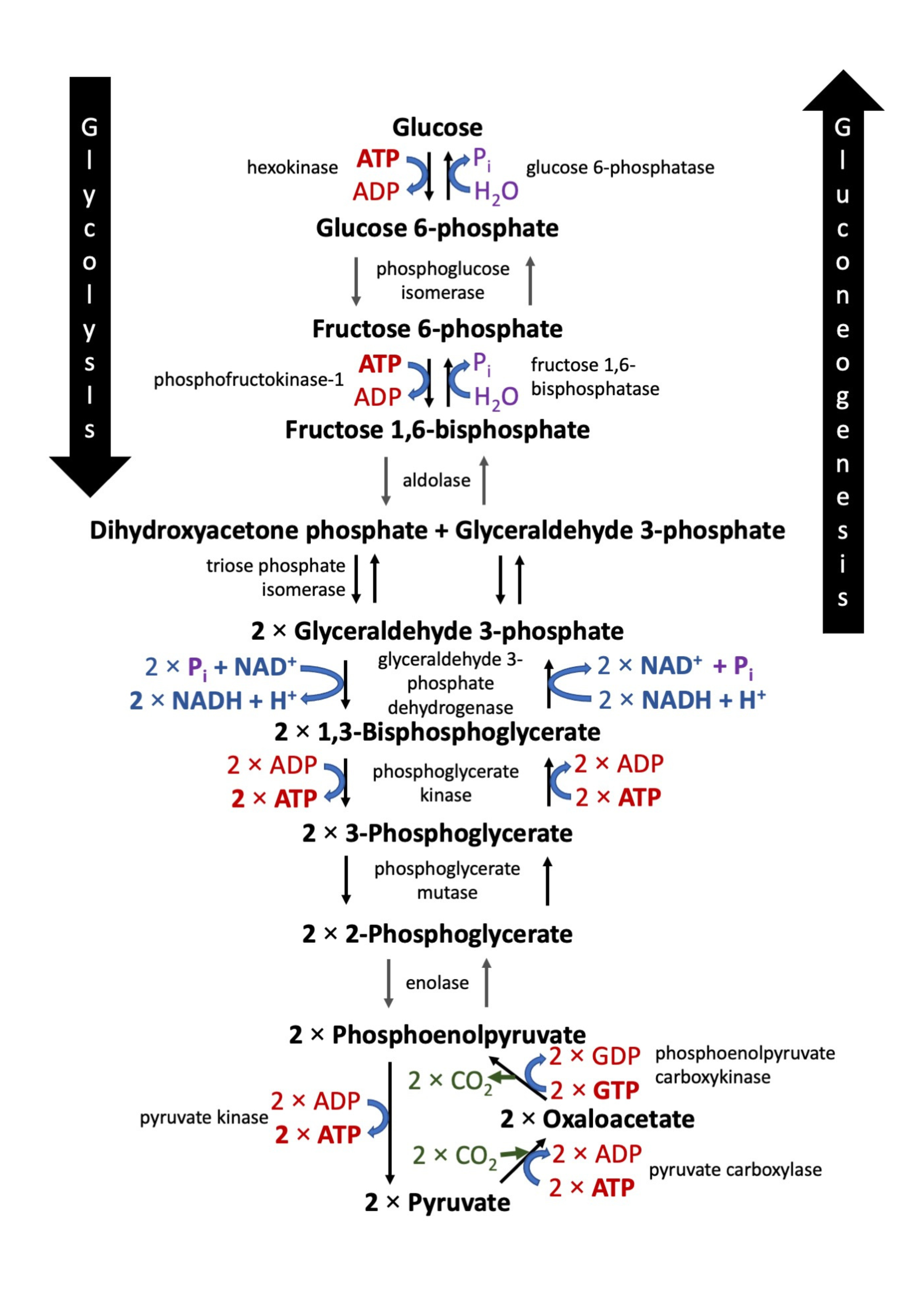
Gluconeogenesis is essentially the reverse process of glycolysis. It generates glucose from non-carbohydrate precursors such as lactate, amino acids, and glycerol. This pathway is vital during fasting or intense exercise, ensuring a continuous supply of glucose to critical tissues like the brain.
The pathway begins with the conversion of pyruvate into oxaloacetate via pyruvate carboxylase, an enzyme that requires ATP and biotin as a cofactor. Oxaloacetate is then decarboxylated and phosphorylated to form phosphoenolpyruvate (PEP) by phosphoenolpyruvate carboxykinase (PEPCK), using GTP as an energy source.
Subsequent reactions reverse most of the glycolytic pathway through a series of enzyme-catalyzed steps until fructose-1,6-bisphosphate is formed. This intermediate is then hydrolyzed by fructose-1,6-bisphosphatase, a key regulatory enzyme in gluconeogenesis, which removes a phosphate group to yield fructose-6-phosphate.
The pathway continues until glucose-6-phosphate is generated, which is then dephosphorylated by glucose-6-phosphatase to produce free glucose that can be released into the bloodstream.
Gluconeogenesis is tightly regulated at both the transcriptional and allosteric levels, with hormones such as glucagon and cortisol upregulating the pathway during periods of low blood sugar, while insulin inhibits it when glucose is abundant.
The pentose phosphate pathway (PPP) operates parallel to glycolysis and fulfills two key functions:
The net molecular and energetic outcomes of these respiration processes are tightly coordinated through intricate metabolic regulation. Cells maintain a dynamic steady state by controlling pathway fluxes through mechanisms such as allosteric regulation, hormonal signaling, and genetic control. For example, key enzymes in glycolysis and gluconeogenesis are regulated in response to energy levels, ensuring that when one pathway is active, the other is suppressed. Similarly, the metabolism of glycogen is finely tuned by enzymes that control both its synthesis and breakdown, allowing organisms to store energy when abundant and mobilize it when needed.
Metabolic pathways are typically governed by rate-limiting steps, where the slowest or earliest irreversible reactions serve as primary control points. Regulation may be positive (amplifying a process) or negative (suppressing it), often forming feedback loops that help maintain a dynamic steady state (homeostasis) in living organisms.
Glycolysis, which converts glucose into pyruvate, is driven by crucial enzymes such as hexokinase, phosphofructokinase, and pyruvate kinase. When the cell has abundant ATP, glycolysis slows, favoring gluconeogenesis instead—essentially a reversal of glycolysis that synthesizes glucose. Conversely, high ADP levels (and low ATP) stimulate glycolysis. Hormones like epinephrine trigger muscle glycolysis and increase blood glucose, while fructose-2,6-bisphosphate promotes glycolysis and downregulates gluconeogenesis.
Glycogen metabolism highlights another layer of regulation. Glycogen breakdown yields glucose-1-phosphate, which can be channeled into multiple pathways (e.g., glycolysis, pentose phosphate pathway) after converting to glucose-6-phosphate. Hormones like glucagon and epinephrine can initiate a cAMP cascade that enhances glycogen phosphorylase activity (breaking down glycogen) and inhibits glycogen synthase (building glycogen). Conversely, lowered cAMP levels shift the balance to glycogen synthesis.
Sign up for free to take 13 quiz questions on this topic