Understanding the absolute configuration of amino acids is fundamental to studying their stereochemical behavior in protein structures and biochemical reactions.
Absolute configuration describes the precise three-dimensional arrangement of substituents around a chiral center—typically the alpha carbon—in an amino acid. When assigning absolute configuration (R or S) to an amino acid, chemists use the Cahn-Ingold-Prelog priority rules to rank the four substituents around the alpha carbon.
In a typical amino acid, the alpha carbon is connected to an amino group, a carboxyl group, a hydrogen atom, and a variable side chain often referred to as R. After prioritizing these four groups, one examines their orientation around the chiral center to assign an R (“rectus”) or S (“sinister”) designation.

This S or R label is strictly about the spatial arrangement of the groups and remains distinct from the D or L notation, which is historically based on comparing the molecule’s projection to that of glyceraldehyde. Biologically, most naturally occurring amino acids are the L form and nearly all of them are S in their absolute configuration (when viewed according to the Cahn-Ingold-Prelog system).
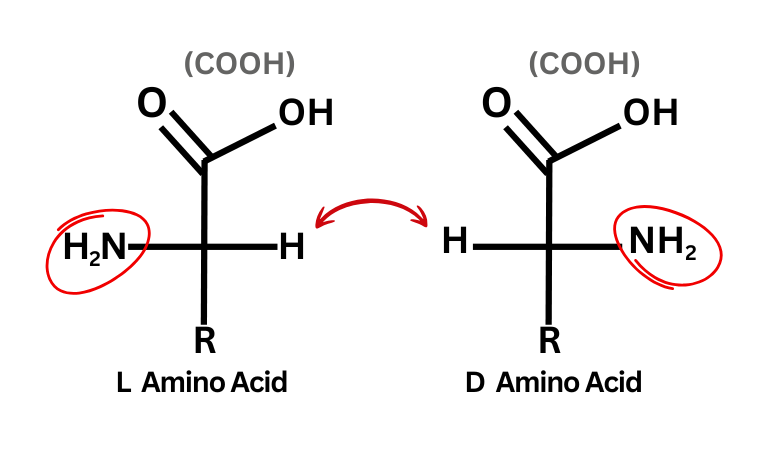
Notable exceptions include glycine, which is achiral as its side chain is simply a second hydrogen, and cysteine, which typically has R configuration because the sulfur-containing side chain outranks the other groups in the priority rules.
Case 1: Priority of
For most amino acids, the substituent ranking is:
Viewed from the correct orientation (with the lowest-priority group—H—pointing away), this arrangement typically leads to an S absolute configuration when the amino acid is in its biologically common L form. Consequently:
Example:
Here, the amino group outranks the carboxyl group, which in turn outranks the side chain. Since the side chain (for instance, a methyl group in alanine) has a lower atomic number priority than the carboxyl group, the final three-dimensional arrangement is S for the L enantiomer.
Case 2: Priority of
For cysteine, the sulfur-containing side chain outranks the carboxyl group because sulfur has a higher atomic number than the oxygen-based substituent in the carboxyl. Thus:
When you apply the CIP sequence rules to this ordering, the spatial arrangement for the L enantiomer becomes R instead of S. So:
Example:
This difference arises because the sulfur atom elevates the side chain above the carboxyl group in CIP priority. As a result, even though cysteine belongs to the same overall L category (based on its relationship to L-glyceraldehyde), its absolute configuration is R rather than S.
In most amino acids, the L form corresponds to the S configuration (Case 1), but cysteine (Case 2) is the classic example where the L form is actually R by CIP rules because of the higher priority sulfur substituent.
Amino acids can exist as dipolar ions, commonly referred to as zwitterions, because each molecule carries both a positive and a negative charge under typical physiological conditions. More specifically, an amino acid contains an amino group (–) and a carboxyl group (–). In an acidic environment, the amino group gains a proton, becoming –⁺, while the carboxyl group often donates a proton, turning into –.
Consequently, the same molecule bears both a positively charged group (on nitrogen) and a negatively charged group (on oxygen) at the same time. This arrangement stabilizes the overall structure and influences how:
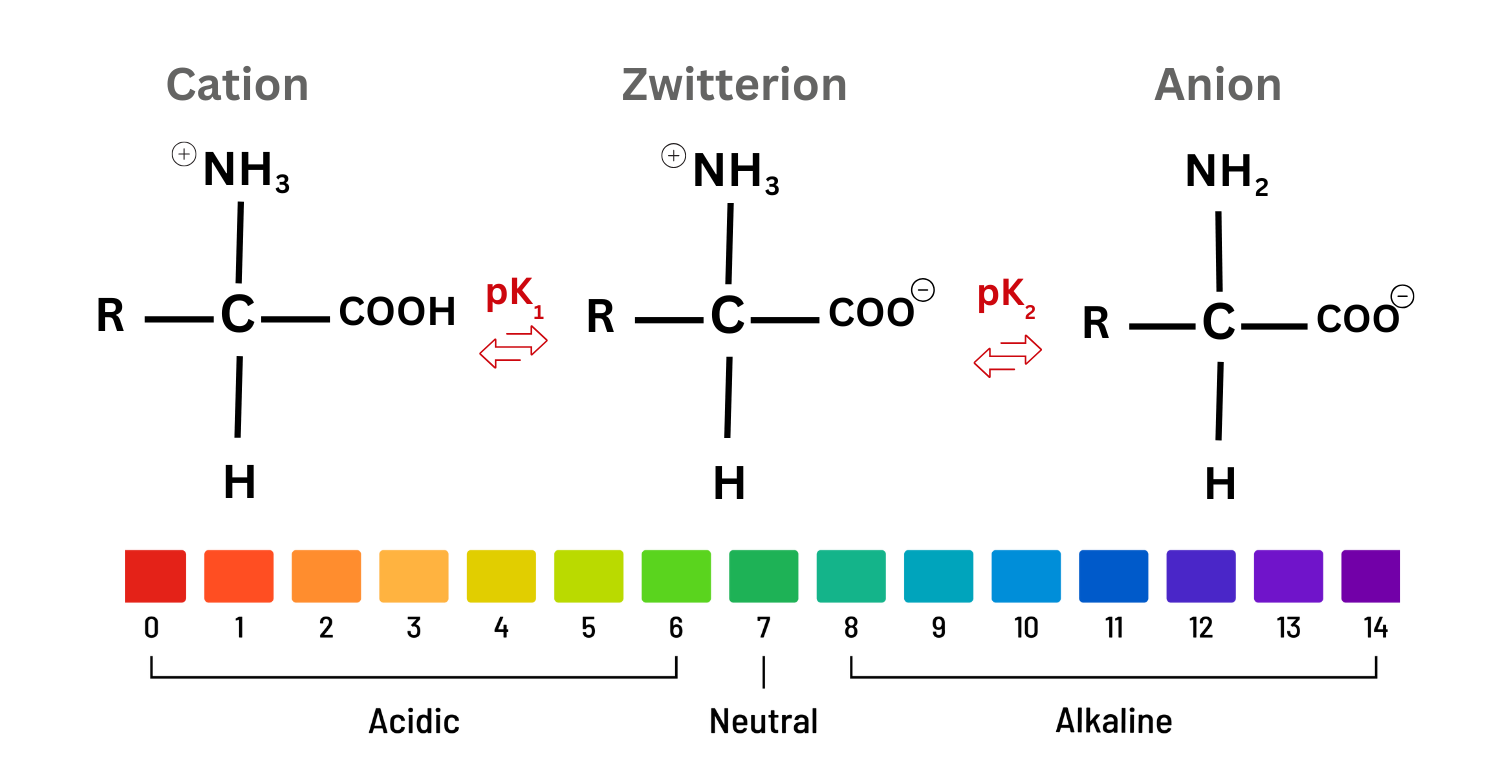
The exact pH value at which an amino acid predominantly assumes its dipolar form varies, based on factors such as side-chain properties and the pKa values of the functional groups. However, in biological systems near neutral pH, most amino acids exist largely in this zwitterionic state, affecting how they behave in aqueous solutions and how they bind to enzymes or other biomolecules.
Acidic or basic
Hydrophobic or hydrophilic
Hydrophobic amino acids typically possess nonpolar side chains consisting mostly of carbon and hydrogen, causing them to avoid water and favor interactions with other nonpolar surfaces. In contrast, hydrophilic amino acids have polar or charged side chains that readily form hydrogen bonds or electrostatic interactions with water. This distinction in side-chain polarity strongly influences:
Sulfur linkage for cysteine and cystine
Peptide linkage: polypeptides and proteins
Hydrolysis
If we denote a compound as AB, where A and B represent atoms or groups, and water is expressed as HOH, the process of hydrolysis can be depicted by the reversible chemical equation:
Sign up for free to take 6 quiz questions on this topic