Enzymes are specialized proteins that act as biological catalysts, greatly accelerating chemical reactions in living organisms by lowering the activation energy. In doing so, they facilitate vital processes such as the following, all at temperatures and pH levels compatible with cellular life:
Enzymes DO:
Enzymes DO NOT:
Composition
Enzymes are often grouped according to the type of reaction they catalyze. Examples include
Each enzyme selectively binds one or more substrates—the molecules undergoing reaction—based on a precise three-dimensional complementarity. This specificity stems from structural compatibility between the substrate and the enzyme’s active site, promoting the correct alignment and efficient chemical conversion. Enzymes typically exhibit high specificity, recognizing subtle differences in substrate shape or functional groups, even as specifically as differentiating stereoisomers.
Enzymes employ diverse strategies to facilitate reactions:
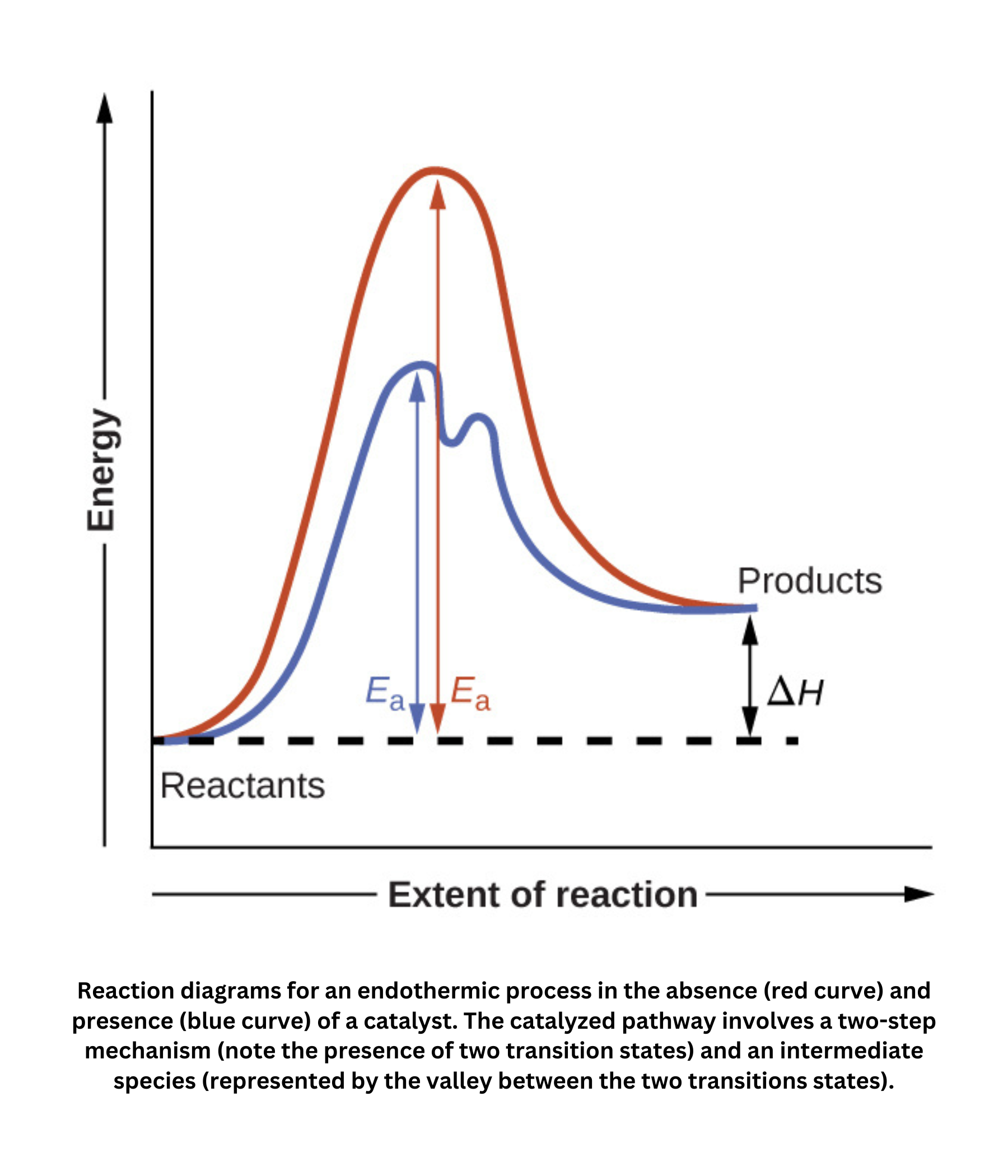
Enzyme activity can be profoundly influenced by environmental factors such as pH, temperature, and concentrations of substrates or products. Each enzyme operates optimally within a specific pH range and temperature window.
Deviations can impair hydrogen bonding or disrupt the protein’s folding, leading to decreased activity or denaturation.
In cells, compartmentalization and regulatory molecules further refine these conditions, ensuring enzymes function at the proper pace and location.
Enzyme activity is governed by a variety of mechanisms that fine-tune how rapidly a catalyzed reaction proceeds, ensuring cells can adapt to changing conditions.
One key area of study is kinetics, which investigates how reaction rates respond to shifts in substrate concentration, enzyme concentration, and other factors. A classic model used to describe these relationships is the Michaelis–Menten framework, which characterizes how an enzyme’s velocity evolves with increasing substrate levels until reaching a maximum rate.
Certain enzymes exhibit cooperativity, meaning the binding of a substrate to one subunit or site affects the affinity of other subunits or sites, producing a more dynamic response curve.
Beyond these intrinsic kinetic properties, feedback regulation offers a means to modulate enzyme function in broader pathways. In this scenario, the end product of a pathway binds upstream enzymes—often the first committed step—to slow or halt its own production. This negative feedback loop helps maintain metabolic balance.
An additional layer of control comes from inhibition, wherein molecules bind the enzyme to reduce or prevent its activity:
Some regulatory enzymes have specialized structural features or undergo modifications that alter their catalytic behavior.
Allosteric enzymes contain sites separate from the active site, where binding of an effector molecule modifies the enzyme’s shape and function, either activating or inhibiting it.
Others are covalently-modified by processes such as phosphorylation, methylation, or acetylation, which can promptly switch an enzyme on or off.
A zymogen represents an inactive precursor form of an enzyme, requiring a specific biochemical alteration—like cleavage of a peptide fragment—to become active.
Sign up for free to take 7 quiz questions on this topic