The ovaries secrete estrogens (primarily estradiol), progesterone, inhibin, and relaxin.
The adrenal gland lacks the enzyme 17 beta hydroxysteroid dehydrogenase, so it can’t synthesize estrogen and testosterone. In contrast, 17 beta hydroxysteroid dehydrogenase is present in the testis and in granulosa cells of the ovaries.
In the ovary:
Both FSH and LH stimulate different steps in estrogen synthesis:
CYP11A1 is also called cholesterol desmolase, cholesterol side chain cleavage enzyme, or P450scc. It’s located in the inner mitochondrial membrane.
Role of activins and inhibins: They are members of the TGF- beta family with opposing actions on FSH secretion and various other biological processes. Activins augment the gonadotropin-releasing hormone (GnRH)-mediated release of FSH while inhibins inhibit FSH release. The gonads are the major sources of circulating activins and inhibins that provide regulatory feedback to the pituitary, and function as autocrine and paracrine signals that control gonadal function.
Role of FSH and LH: Both FSH and LH are glycoprotein hormones released by the anterior pituitary in response to GnRH. They are also called gonadotrophins. They act via GPCRs. Receptor activation by gonadotrophin binding stimulates adenylate cyclase and a consequent rise in cAMP.
LH and FSH increase intracellular concentrations of free cholesterol, its transport to the mitochondria, and the transport of cholesterol to the inner mitochondrial membrane by the StAR protein. The rate-limiting step in gonadotrophin-induced steroid synthesis is the regulation of StAR and conversion of cholesterol to pregnenolone by enzyme CYP11A1.
In the testis, LH acts on the interstitial or Leydig cells while FSH acts exclusively on the Sertoli cells. In the ovary, both LH and FSH are involved in the control of steroidogenesis and each acts on more than one cell type.
Both FSH and LH are secreted in a pulsatile fashion, mirroring GnRH pulses. Ovarian granulosa cells have FSH receptors. FSH stimulates the growth of the granulosa cells in the primary follicles and the production of estradiol. LH, along with FSH, stimulates estradiol synthesis.
Levels of LH rise sharply just before ovulation, leading to rupture of the dominant follicle and ovulation. LH supports the corpus luteum. Receptors for LH are located on the corpus luteum.
GnRH: It is produced in the hypothalamus and transported to the gonadotrophs of the anterior pituitary by the hypothalamo-hypophyseal portal capillaries. GnRH is secreted in a pulsatile manner, with a single pulse occurring approximately hourly.
GnRH acts through GPCR and activates Gq to increase intracellular Ca++, DAG, and IP3. GnRH stimulates both the synthesis and release of LH and FSH.
The amplitude and frequency of GnRH pulses influence the actions of GnRH:
Continuous (non-pulsatile) infusions of GnRH cause an initial stimulus to gonadotrophin secretion followed by a down-regulation of GnRH receptors and abolition of FSH and LH release.
GnRH levels remain low until the onset of puberty, which is characterized by onset of pulsatile secretion of GnRH. FSH and LH follow the pattern of GnRH at puberty.
Menstrual cycle: The cyclical activity of the ovaries is manifested as the menstrual cycle. It is regulated by a complex mechanism involving positive and negative feedback cycles between the ovarian hormones, GnRH, FSH, and LH.
A typical menstrual cycle lasts for 28 days and is divided into two phases: follicular and luteal. The menstrual cycle can vary from 21 to 35 days. This variability is due to changes in the length of the follicular phase, because the luteal phase is constant.
The follicular period precedes ovulation. It is dominated by estrogen, which stimulates the growth of the endometrium, glands, stroma, and spiral arteries, so it’s also called the proliferative phase.
Under the effect of estrogen, cervical mucus becomes copious, watery, and elastic and shows a classic “ferning” pattern, which is conducive to penetration by sperm.
In this phase, a primordial follicle develops into a Graffian follicle and then into a dominant follicle. FSH and LH receptors are upregulated in the theca and granulosa cells, resulting in increased levels of estradiol.
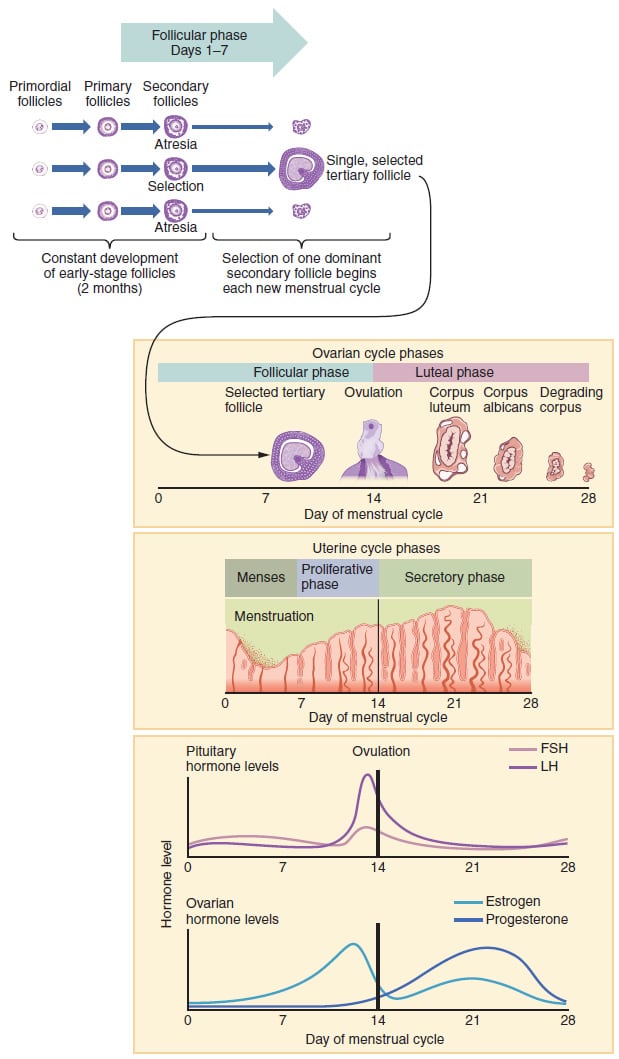
Towards the end of the follicular phase, there is an estrogen surge that increases FSH and LH secretion from the anterior pituitary by positive feedback. Ovulation occurs 9-12 hours after the LH surge and involves local paracrine mechanisms and induction of proteolytic enzymes. This is followed by a temporary decrease in estrogen levels, which rise again shortly after in the luteal phase.
The 14-day period that follows ovulation is called the luteal (secretory) phase and is dominated by progesterone. During this phase:
The corpus luteum develops and secretes both estradiol and progesterone. Progesterone increases the basal body temperature.
If there is no pregnancy, the corpus luteum degenerates, leading to a drop in estrogen and progesterone. This drop causes menstruation. The first day of menstruation is taken as day 1 of the menstrual cycle.
Loss of negative feedback by estrogen and progesterone leads to a rise in FSH, which initiates the follicular phase.
The predominant mechanism of estrogen action is through nuclear estrogen receptor (ER) expression in target organs. The biological effects of estrogen are mediated through two distinct ER proteins: ERα and ERβ. These receptors are encoded by separate genes on different chromosomes.
Estriol is the major form of estrogen in pregnancy. Estrones are the estrogen type seen after menopause.
Progesterone effects are mediated by its nuclear receptor, PR. It functions as a regulator of nuclear transcription. Estrogen increases the expression of PR in most tissues.
Sign up for free to take 20 quiz questions on this topic