Necrotic cells become more eosinophilic and often have a homogeneous, glassy-appearing cytoplasm. After enzymatic digestion, the cytoplasm may look vacuolated. Myelin figures may appear; these are whorled phospholipid masses seen after necrosis. Local calcification may follow.
On electron microscopy, you may see:
Nuclear changes include pyknosis, karyorrhexis, and karyolysis.
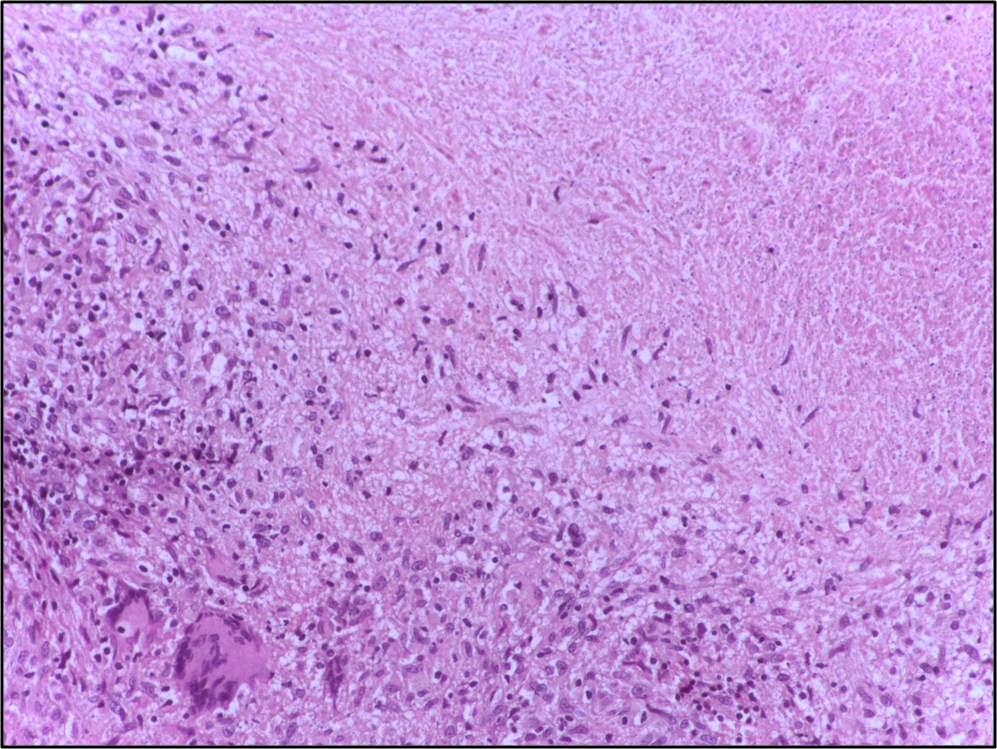
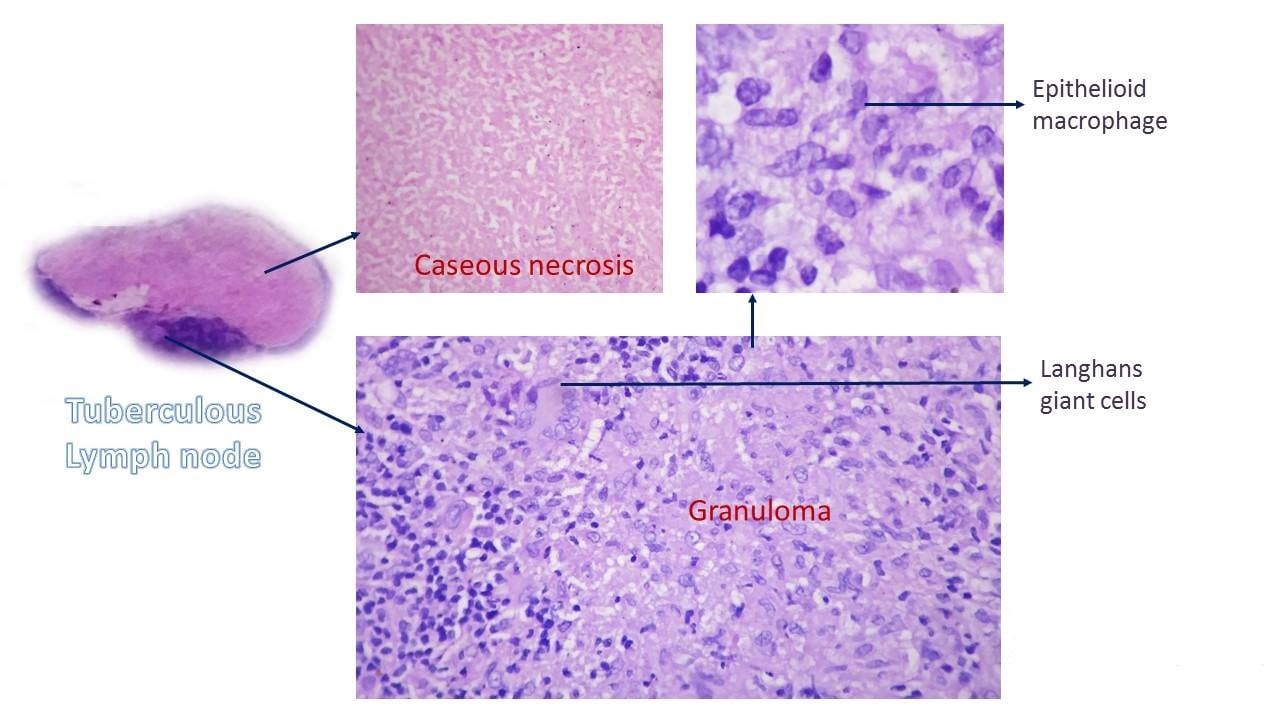
Types of necrosis
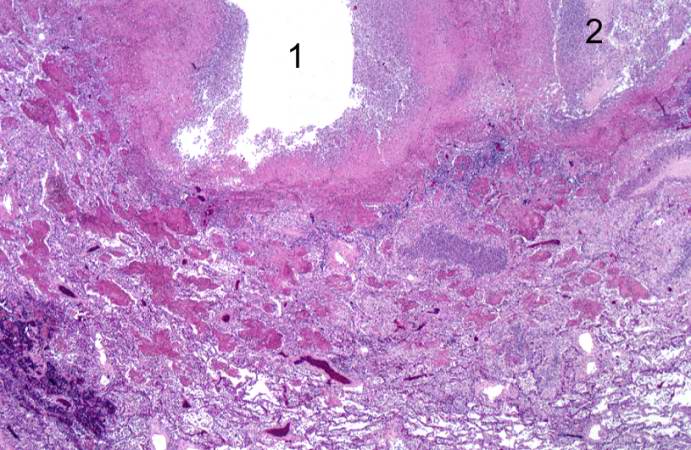
This higher-power photomicrograph of the lung demonstrates the edge of the abscess. Note the loss of material from the center of the abscess (1) and loose necrotic material that has not been expelled (2). This material is made up of inflammatory cells (primarily dead white blood cells) and necrotic lung tissue.
Fat necrosis: It is seen in the setting of acute pancreatitis with the release of activated pancreatic lipases, which cause necrosis of the pancreas and surrounding peritoneal structures. The lipases also break down stored triglycerides to release free fatty acids, which combine with calcium to form chalky white soap deposits in the pancreas, a process called fat saponification. Calcium deposits appear basophilic on microscopy.
Fibrinoid necrosis: It is a special type of necrosis seen in small blood vessels like muscular arteries, arterioles and glomeruli in vasculitis associated with autoimmune diseases like SLE and in malignant hypertension. The vessel walls show fibrin deposits and appear homogeneously red or eosinophilic.
Response of subcellular organelles to injury: Heterophagy is the digestion of extracellularly ingested materials by lysosomes. It is seen in phagocytes like neutrophils and macrophages. Autophagy is the digestion of a cell’s own components by lysosomes. Auto-phagolysosomes are seen in autophagy. It is associated with atrophy, fasting, removal of damaged cell components, and tissue remodeling. Carbon particles from inhaled air or carbon pigment from tattoo ink can persist in the phagolysosomes of macrophages for decades. Chloroquine raises the pH inside the lysosome, inactivating lysosomal enzymes. Certain drugs and toxins, like barbiturates, alcohol, etc., can induce the smooth endoplasmic reticulum (SER) and cause SER hypertrophy. Mitochondria may abnormally swell into large, irregular shapes and sizes called megamitochondria as a result of free radical injury associated with alcoholism, hydrazine, and chloramphenicol. Large mitochondria with abnormal cristae are seen in mitochondrial myopathies.
| Actin | Phalloidin from mushroom Amanita phalloides binds and inhibits actin |
| Microtubules | Defective in Kartagener’s syndrome (immotile cilia), Colchicine and Vinca alkaloids bind to tubulin and prevent assembly of the mitotic spindle; Paclitaxel interferes with disassembly of mitotic spindles |
| Keratin* | Mallory bodies in alcoholic liver disease |
| Neurofilaments* | Neurofibrillary tangles in Alzheimer’s disease; Lewy bodies in Parkinson’s disease |
| Vimentin* | Colon cancer, esophageal cancers, thyroid cancers |
| Desmin* | Cardiomyopathies |
| Glial filaments* | Neuronal cell adhesion and signalling pathways increased in astrocytomas |
*Keratin, neurofilaments, desmin, vimentin, and glial filaments are also called intermediate filaments.
Section D: Intracellular accumulations
| Accumulations of endogenous substances - fat | ||
| Fatty liver | Triglycerides. Appears as vacuolated cells in H and E sections and orange-red in Sudan IV or oil-red stains. | Seen in metabolic syndrome, insulin resistance, alcoholic liver disease, CCl4 poisoning, malnutrition, anoxia, starvation |
| Fatty change in the heart | “Tiger heart” with alternating bands of yellow (fatty change) and normal red-brown myocardium | Hypoxia, severe anemia, diphtheria |
| Cholesterol accumulation | Cholesterol laden macrophages and smooth muscle cells in vessel intima called “foam cells”, needle-shaped cholesterol clefts; foam cells in macrophages in skin and tendons; | Atherosclerotic plaques, xanthomas; inflammatory and necrotic areas; gall bladder in cholesterolosis; xanthelasma Niemann-Pick disease type C. |
| Accumulations of endogenous substances - proteins | ||
| Amyloidosis | Different types of amyloid proteins derived from light chains, APP, etc. Stains orange-red with Congo red and shows green birefringence under polarised light. | Monoclonal gammopathy, chronic inflammation , etc. |
| Increased apoptosis due to misfolded proteins | Misfolded proteins accumulate in the ER and trigger caspases | Neurodegenerative disorders like Alzheimer’s disease, Parkinson’s disease |
| Defects in protein transport and secretion | Abnormally folded proteins with loss of function | Cystic fibrosis, Alpha 1 antitrypsin deficiency, Familial hypercholesterolemia |
| Russel bodies | Eosinophilic inclusions in the endoplasmic reticulum of plasma cells (Mott cells) resulting from immunoglobulin synthesis | Multiple myeloma, inflammatory and cancerous colon polyps |
| Lysosomal storage disorders | Defect in the breakdown of macromolecules like lipids, glycosaminoglycans, etc, by lysosomes; cytoplasmic inclusions seen | Niemann Pick disease, Gaucher’s disease etc. |
| Glycogen storage disorders | Glycogen or limit dextrins accumulate in the cytoplasm | Von -Gierke’s disease; Pompe’s disease etc. |
| Endogenous pigments | Lipofuscin - yellow-brown, intracytoplasmic or perinuclear pigment seen in liver, heart in aging, malnutrition, cancer cachexia; Melanin - melasma, solar lentigines, acanthosis nigricans, Peuts Jeghers syndrome, Addison’s disease (from MSH like activity of ACTH); Homogentisic acid - black pigment deposits in cartilage in alkaptonuria; Hemosiderin - bruises, hemosiderosis in iron overload, hemochromatosis | |
| Accumulation of exogenous substances | ||
| Carbon | Carbon particles are engulfed by macrophages (dust cells) | Anthracosis, coal worker’s pneumoconiosis, tattoos |
| Lead | Acid-fast intranuclear lead deposits in PCT cells | Lead poisoning |
Sign up for free to take 11 quiz questions on this topic