Bioenergetics investigates how energy flows and transforms within biological systems. This concept underlies many cellular functions, from metabolism to biosynthesis.
Thermodynamics frames how enthalpy (H), entropy (S), and free energy (G) govern a reaction’s likelihood. The equation
reveals whether a process is spontaneous (negative G) or nonspontaneous (positive G).
The equilibrium constant () quantifies the ratio of products to reactants at equilibrium. Under standard conditions, the relationship between free energy and equilibrium is:
Changing concentrations of reactants or products shifts equilibrium according to Le Châtelier’s Principle. By altering levels of a species in the reaction, cells can push a reaction forward or backward to maintain metabolic balance.
In cells, ATP (adenosine triphosphate) provides a readily accessible energy currency. Phosphorylation transfers a phosphate group to substrates or enzymes, altering their reactivity or function in countless biochemical pathways.
Organisms frequently harvest energy via redox reactions, transferring electrons from nutrients to electron acceptors. The energy liberated fuels ATP production and other endergonic steps in metabolism.
In chemical reactions, energy changes can manifest as either endothermic or exothermic processes. Endothermic reactions absorb heat from their surroundings (), while exothermic reactions release heat (). Standard heats of reaction and formation provide quantitative measures of these energy changes under specified conditions, indicating whether heat is required or liberated during the transformation.
Enthalpy (H) represents the total heat content of a system at constant pressure. The change in enthalpy () during a reaction is measured under standard conditions, often expressed in joules per mole. Standard heats of reaction () reflect the net energy change when reactants are converted to products, while standard heats of formation () represent the energy change when one mole of a compound forms from its elements in their standard states.
Hess’s Law states that the total enthalpy change for a reaction is independent of the pathway taken. This principle allows the enthalpy change for complex reactions to be computed by summing the enthalpy changes of individual steps, expressed as:
The spontaneity of a reaction is determined by its free energy change (). A reaction with a negative is thermodynamically favorable, meaning it proceeds spontaneously under the given conditions. It is important to note that while exothermic reactions (negative ) often have negative values, the overall spontaneity also depends on the change in entropy () of the system, as described by the relationship:
In biological systems, energy transfer is frequently driven by the hydrolysis of ATP. ATP hydrolysis is highly exergonic (), releasing energy that can be used to drive phosphoryl group transfers. These transfer reactions are crucial for activating molecules, regulating metabolic pathways, and powering various cellular functions.
Cellular energy production depends on oxidation–reduction (redox) reactions, where electrons are transferred between molecules. These reactions occur in a series of half-reactions and are mediated by soluble electron carriers such as and . Flavoproteins and other enzymes facilitate these electron transfers, playing a central role in the bioenergetic pathways that convert chemical energy into a form that cells can use.
A thermodynamic system is characterized by state functions—properties like temperature, pressure, volume, and internal energy—that depend solely on the current state of the system rather than on the path used to arrive there.
The zeroth law establishes that if two systems are each in thermal equilibrium with a third, they are in thermal equilibrium with one another. This foundational principle defines temperature as the means by which heat exchange is governed.
The first law expresses the conservation of energy, stating that the change in a system’s internal energy equals the heat added minus the work done by the system. Energy is thus neither created nor destroyed; it only transforms.
In a pressure–volume (PV) diagram, the work performed by a system during expansion or compression is represented by the area under or enclosed by the process curve. This graphical depiction helps visualize energy transfer through work.
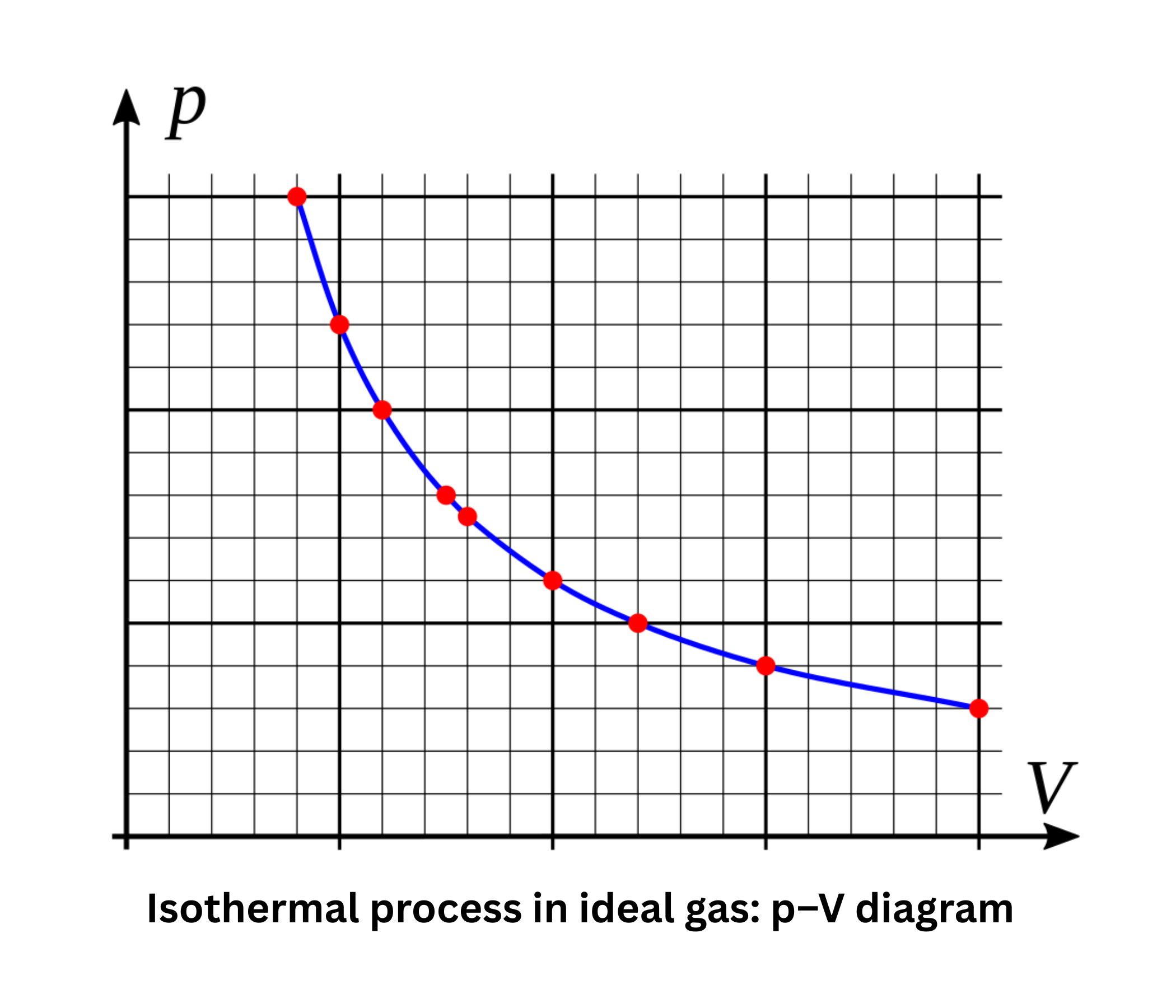
The second law introduces entropy as a measure of disorder or randomness. It asserts that in any natural process, the total entropy of an isolated system tends to increase, driving the irreversibility of spontaneous changes.
Entropy quantifies molecular disorder. Generally, gases possess high entropy due to their molecular freedom, liquids have moderate entropy, and crystalline solids exhibit low entropy because of their highly ordered structures.
Different states of matter display distinct entropy levels: gases have the highest entropy because their molecules are widely dispersed, liquids are intermediate, and crystals are the most ordered with the lowest entropy.
Calorimetry is the experimental technique used to measure heat changes in reactions. Heat capacity is the amount of heat required to raise the temperature of a system, while specific heat refers to the heat needed per unit mass for a one-degree temperature change.
Heat can be transferred via conduction (direct molecular collisions), convection (bulk movement of fluids), and radiation (emission and absorption of electromagnetic energy). These processes describe how energy is dispersed in different mediums.
Bond dissociation energy, the energy required to break a chemical bond, is closely tied to the heats of formation of compounds. High bond energies indicate more stable molecules, influencing the overall energy change during chemical reactions.
The spontaneity of a reaction is determined by the standard free energy change, . A negative suggests that the reaction occurs spontaneously under standard conditions, whereas a positive value indicates nonspontaneity.
The coefficient of expansion measures how the volume or length of a material changes with temperature. Materials with higher coefficients expand more significantly when heated, impacting both engineering applications and thermodynamic calculations.
The heat of fusion is the energy required to change a substance from solid to liquid at its melting point, and the heat of vaporization is the energy needed to convert a liquid into a gas at its boiling point. Both values reflect the energy needed to overcome intermolecular forces during phase transitions.
A phase diagram maps the equilibrium between different phases of a substance across varying pressures and temperatures. It highlights critical conditions such as the triple point, where solid, liquid, and gas coexist, and the critical point, where the distinction between liquid and gas phases disappears.
Sign up for free to take 6 quiz questions on this topic