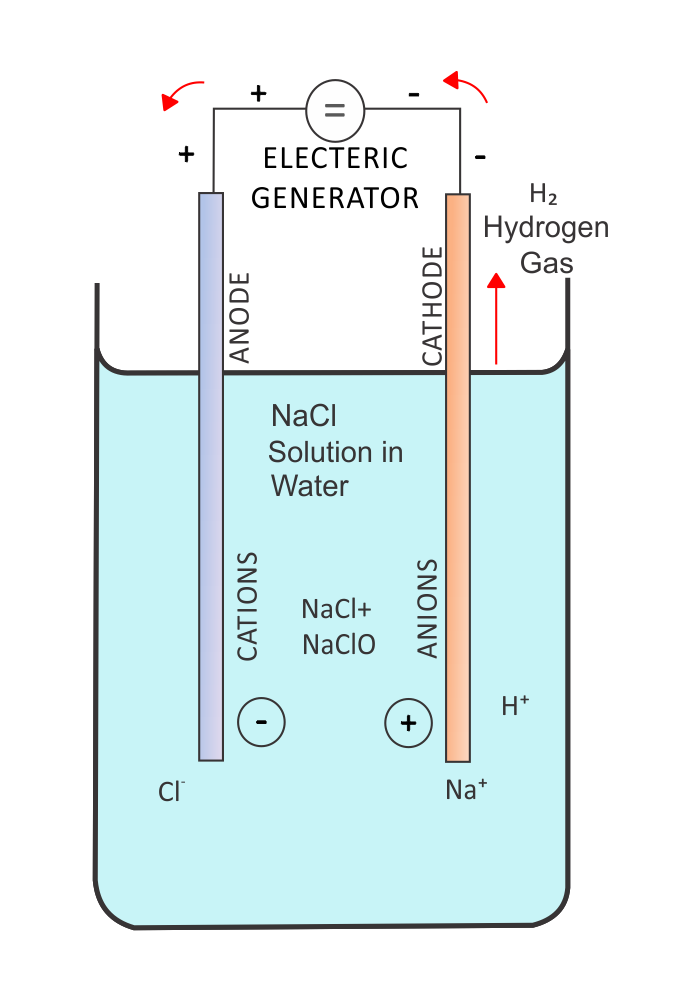
Electrolysis is a process where an external voltage source, typically a battery, is used to drive chemical reactions that would not occur spontaneously.
In an electrolytic cell, the inherent cell potential is negative, so the applied voltage must be greater than this magnitude to force the reaction forward. This external voltage overcomes the natural tendency of the electrons to flow in the opposite direction.
In contrast, a galvanic cell (or voltaic cell) naturally produces a positive cell potential and does not require an external voltage source. In diagrams, red arrows often indicate the forced electron flow due to the battery, emphasizing that the electrons are moving contrary to their spontaneous direction under normal conditions.
-cells--20250718135943--f472808ae8327ebbff0064c0ad386b422aa0fb4c065aaecc02313908a2318a54.png)
In both electrolytic and galvanic cells, the anode is where oxidation occurs, meaning it is the site that releases electrons, while the cathode is where reduction happens, absorbing electrons.
Additionally, these cells rely on an electrolyte, a medium containing ions that facilitate the flow of electricity by moving through the solution. Without an electrolyte, the circuit cannot function because there are no mobile charge carriers to complete the electrical path.
Faraday’s law relates the amount of substance deposited at an electrode to the current flowing through the system. Current is defined as the charge transferred per unit time ().
Faraday’s constant represents the charge per mole of electrons, so that . Since can also be expressed as , equating these expressions gives I·t = nF. This equation means that the product of current and time equals the number of moles of electrons transferred times Faraday’s constant. Using this relationship along with the stoichiometry of a half-reaction, one can determine the number of moles of an element deposited for every electron transferred. For example, in the reaction → , one mole of copper is deposited for every 2 moles of electrons.
In an electrochemical cell, electron flow is driven by redox reactions.
At the anode, oxidation occurs, meaning that a substance (M) loses electrons (). These electrons are directed through the external circuit toward the cathode, where reduction takes place ().
This means oxidation increases charge by removing electrons, while reduction decreases charge by adding electrons.
A galvanic cell (or voltaic cell) converts chemical energy into electrical energy through a spontaneous redox reaction. In such a cell, two half-reactions occur: one oxidation half-reaction, where a species loses electrons, and one reduction half-reaction, where another species gains electrons.
Each half-reaction is associated with a reduction potential, which quantifies the tendency of a species to gain electrons, while the oxidation potential is simply the negative of the reduction potential for the same reaction.
The overall cell potential is determined by summing the reduction potential at the cathode with the oxidation potential at the anode. A positive cell potential indicates that the cell can produce electric current spontaneously.
For instance, if the cathode reaction is with a reduction potential of and the anode reaction is (with an oxidation potential of ), then the cell potential is , confirming the cell’s spontaneous operation.
In any electrochemical system, electrons always flow from the anode to the cathode.
At the anode, oxidation occurs, meaning that a substance loses electrons (and produces cations), which then travel through the external circuit toward the cathode, where reduction takes place as electrons are accepted by another species. A useful mnemonic is “A to C,” reflecting that the anode comes before the cathode alphabetically.
In naturally occurring systems like galvanic cells, the species with the highest oxidation potential (or lowest reduction potential) acts as the anode, while the one with the highest reduction potential serves as the cathode, resulting in spontaneous electron flow.
In contrast, in electrolytic cells, an external battery forces the electron flow, even if this reverses the natural roles of the electrodes. In both cases, the complete circuit is maintained by electrons moving through wires and electrodes, while ions in the electrolyte balance the charge, allowing the redox reactions to continue.
A concentration cell is formed by connecting two nearly identical half-cells, both operating on the same half-reaction and using identical electrodes. The only difference between them is the concentration of one redox species, so the cell’s potential is solely determined by the difference in concentration of that species.
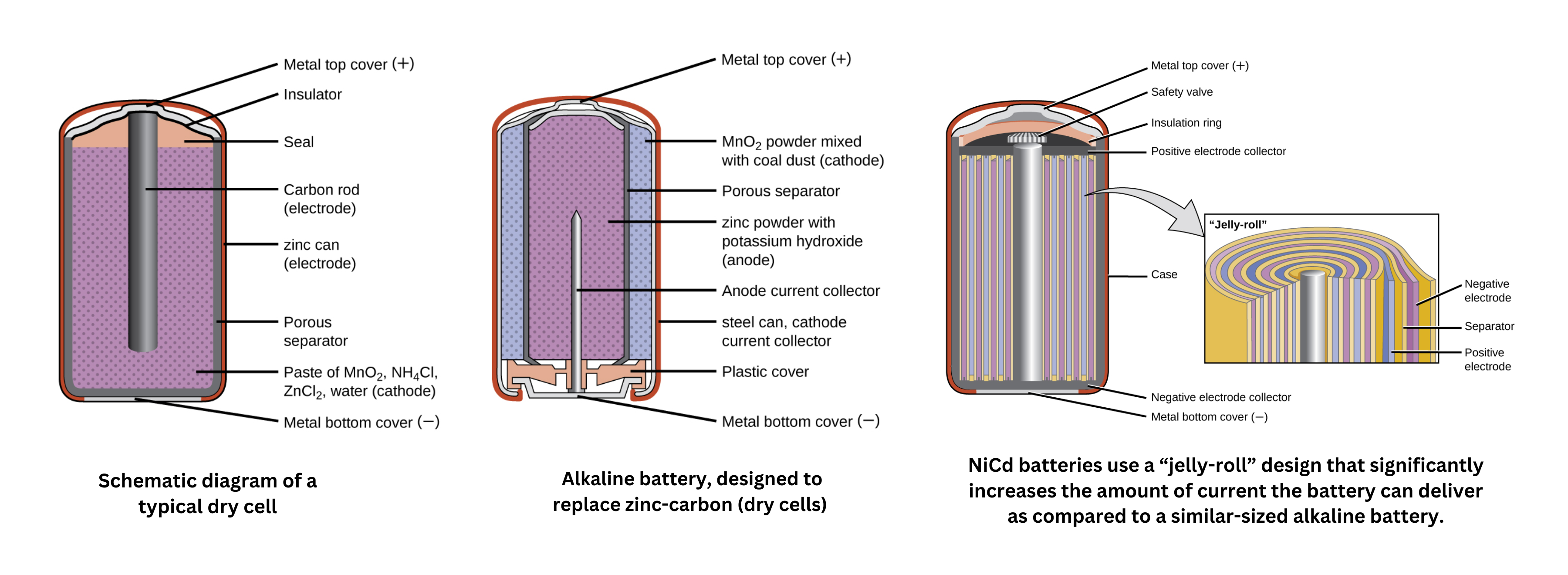
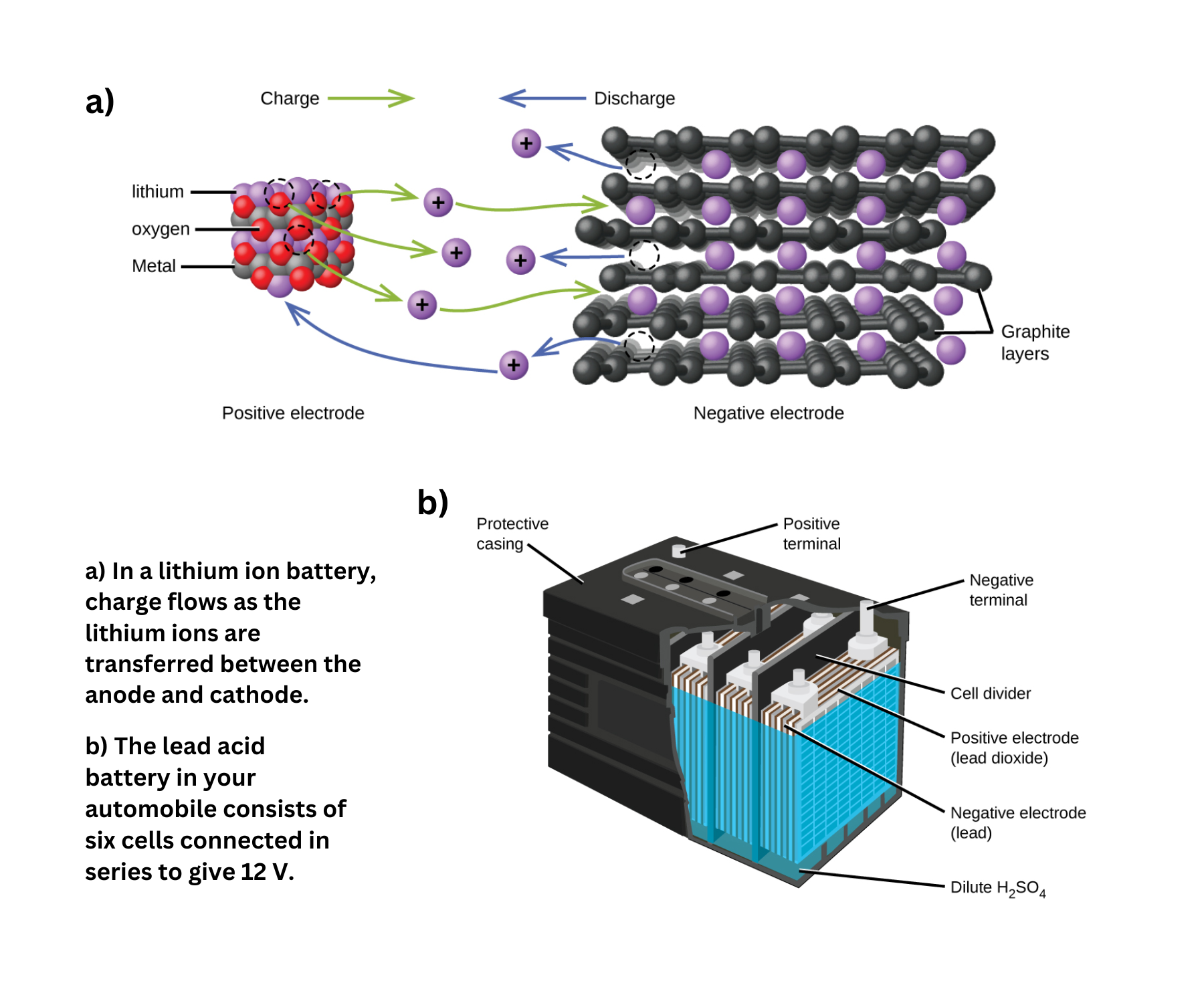
A battery is a specially designed galvanic cell used to provide electrical power for specific applications. A battery is a specially designed galvanic cell used to provide electrical power for specific applications. A battery provides an emf (electromechanical force) that ideally equals the terminal voltage if there is no internal resistance. In real batteries, however, internal resistance causes a voltage drop, resulting in a terminal voltage lower than the emf.
Some common battery types:
Attached to the cell body of nerve cells are dendrites, branching structures that serve as the neuron’s receptive region, boosting surface area for incoming signals.
Extending away from the cell body is a single axon, which conducts electrical impulses toward the axon terminals—sometimes called synaptic knobs or boutons—where neurotransmitters are released.
The axon may be wrapped in a myelin sheath, produced by Schwann cells in the peripheral nervous system or by oligodendrocytes in the central nervous system. This myelin sheath, composed of fatty layers, acts as insulation at intervals along the axon, leaving exposed gaps known as nodes of Ranvier. Because these nodes lack myelin, the action potential jumps from one node to the next, greatly accelerating nerve impulse conduction.
Sign up for free to take 11 quiz questions on this topic