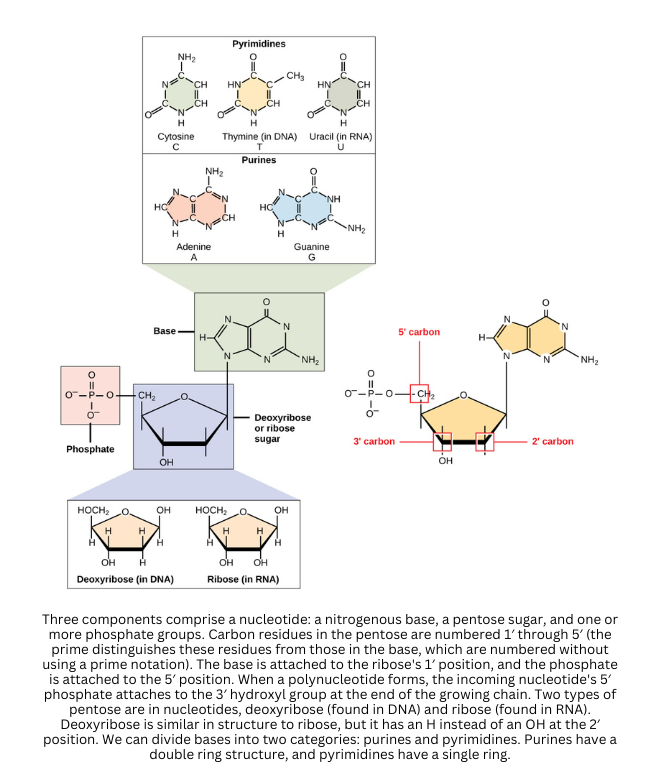
Nucleotides
When multiple nucleotides link together through phosphodiester bonds between their sugar and phosphate groups, they form the sugar-phosphate backbone characteristic of DNA and RNA.
Within this backbone, the nitrogenous bases project inward and can be broadly categorized into purines and pyrimidines:
In DNA—or deoxyribonucleic acid—thymine replaces uracil, forming a molecular chain that is typically double-stranded in most cells. According to the Watson–Crick model, based heavily on images by Rosalind Franklin, DNA is a double helix wherein two complementary strands wrap around a common axis.
DNA’s primary role is the transmission of genetic information across generations. Its sequence of bases encodes instructions that direct cellular processes, while the complementary, antiparallel design allows the strands to serve as templates during replication.
Under conditions such as high temperature or extreme pH, DNA can undergo denaturation, in which the hydrogen bonds break and the two strands unwind. If optimal conditions are restored, reannealing can occur as the complementary bases realign, rebond, and the helix re-forms.
Similarly, hybridization exploits complementarity by allowing single-stranded DNA from different sources—or DNA with RNA—to base-pair when matching sequences are present.
DNA replication is a highly coordinated process by which a cell duplicates its genetic material, yielding two identical copies of double-stranded DNA from one original molecule. This process begins when DNA gyrase helps uncoil the DNA in front of the replication fork, while helicase unwinds the double helix at the fork itself. Helicase unwinds the helix, separating the two strands at specific sites called origins of replication. In eukaryotes, numerous origins are scattered along each chromosome, enabling replication to proceed more quickly. Lastly, Single-strand binding protein (SSB) stabilizes the newly unwound, single-stranded sections of DNA by binding to them and preventing reannealing.
Once unwound, each strand serves as a template for building a new complementary strand. Free nucleotides pair with their corresponding bases on the exposed template according to strict base-pairing rules (A with T, G with C). An important detail is that DNA synthesis in cells always occurs in the 5′ → 3′ direction. Due to the antiparallel nature of DNA, the template strand is always read in the opposite 3′ → 5′ direction.
Primase initiates this process by placing a short RNA primer on the unwound DNA. Although it consists of RNA, this primer base-pairs with the DNA template. Later, the RNA is replaced by DNA. Subsequently, DNA polymerase extends these primers, synthesizing DNA complementary to the template strands.
Both newly unwound strands are copied simultaneously:
Finally, a specialized DNA polymerase removes the RNA primers and replaces them with DNA, after which DNA ligase seals the gaps between Okazaki fragments on the lagging strand.
This replication process unfolds in both directions, forming two replication forks that move outward like an expanding bubble. DNA polymerase possesses a proofreading function that allows it to correct most errors as it works. In eukaryotes, replication takes place once each cell generation during S phase, and although meiosis involves two rounds of cell division, the replication of DNA itself occurs only once.
DNA polymerase enzymes also catalyze the formation of phosphodiester bonds that link nucleotides into a growing chain.
Because each newly formed double helix includes one parental strand and one daughter strand, replication is termed semi-conservative.
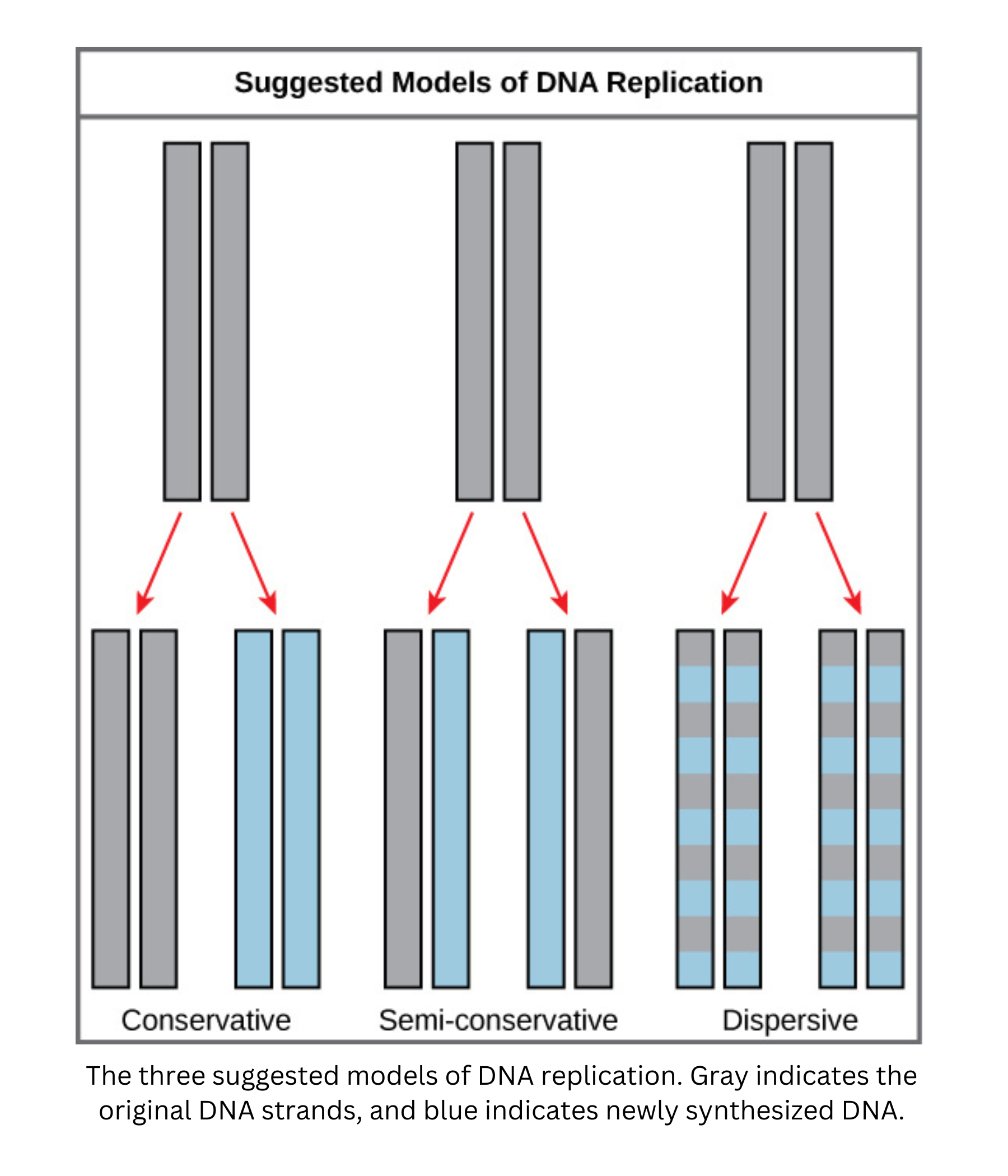
Meselson and Stahl designed an experiment to distinguish between competing models of DNA replication. They grew bacteria in a medium containing a heavy isotope of nitrogen (15N), so that all of the bacterial DNA was labeled with this heavy nitrogen. Then, they shifted the bacteria to a medium containing only the lighter isotope (14N). After one round of replication, they extracted the DNA and subjected it to density gradient centrifugation. Instead of seeing two distinct bands—as would be expected if replication were either conservative (one band for completely heavy DNA and one for completely light DNA) or dispersive (a single band with intermediate density that gradually shifts with each generation)—they observed a single band with an intermediate density. This result indicated that each DNA molecule now consisted of one heavy strand (from the original DNA) and one light strand (newly synthesized), which supports the semi-conservative model of DNA replication.
After a second round of replication, two bands appeared: one still at the intermediate (hybrid) density and a new, lighter band. The presence of the lighter band confirmed that some of the DNA molecules had two new strands, while the hybrid molecules retained one old and one new strand.
The replication forks advance in both directions away from each origin, creating replication bubbles.
A special challenge arises at the ends of linear DNA in eukaryotes, known as telomeres. Over successive rounds of replication, these ends can shorten because DNA polymerase cannot fully replicate the extreme 3′ end of the lagging strand. An enzyme called telomerase extends these telomeric regions, compensating for the end-replication problem and preserving chromosome integrity across multiple cell divisions. Over the course of a lifetime, the gradual shortening of telomeres is associated with physiological aging.
Repair during replication
During DNA replication, DNA polymerase inherently minimizes errors through a proofreading mechanism called 3′→5′ exonuclease activity. When an incorrect nucleotide is inserted, the enzyme detects the mismatch, reverses direction (“backs up”), excises the faulty base, and inserts the correct one.
Another specialized polymerase, responsible for replacing RNA primers with DNA, relies on 5′→3′ exonuclease activity to remove these primers or any short stretches of erroneous nucleotides and substitute them with the proper DNA sequence.
Repair of mutations
Additional processes correct a variety of mutation types encountered outside or in conjunction with replication:
Sign up for free to take 8 quiz questions on this topic