Look-alike, sound-alike medications are drugs whose packaging or appearance (color, size, shape) is similar or whose names, doses, or strengths sound similar. This can lead to errors while ordering, filling, dispensing, or using medications. Such errors are called LASA errors. LASA errors are a type of medication errors.
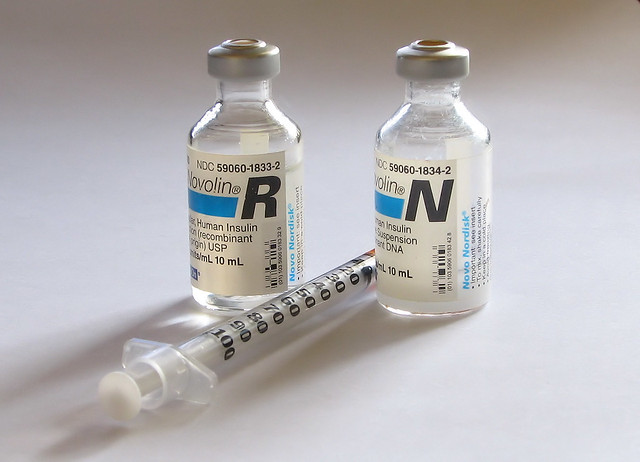
Examples of sound-alike medications are Oxynorm and Oxycontin, Bupropion, and Buspirone. Different strengths of the same medication may sound alike and lead to prescription errors; e.g., Warfarin tablets come in strengths of 1 to 10 mg, which may cause errors and result in over or underdosing, leading to potentially fatal adverse effects. Another example is misidentifying different forms of Insulin, like HumuLIN® R U-500 (Regular insulin human), HumuLIN® N NPH (human insulin isophane), etc.
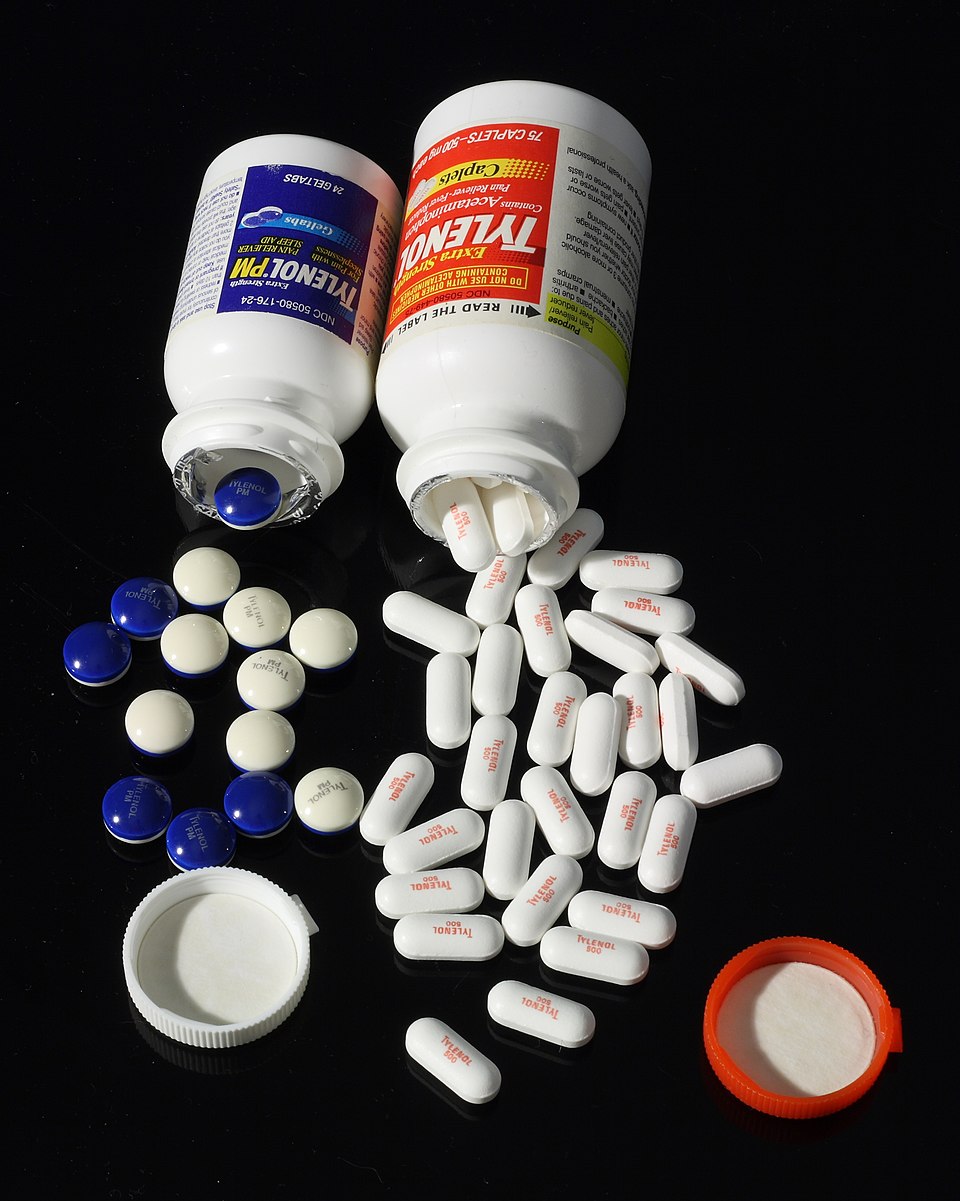
Similar packaging, labeling, and coloring may cause errors in dispensing or intake of look-alike drugs. In previously reported LASA errors, vials of tranexamic acid (used to control bleeding) were confused with either bupivacaine or ropivacaine because the bottles were similar in size and had blue caps. Such errors are especially common while using anesthetic drugs.
LASA errors can have severe outcomes, including overdosing, underdosing, or unintended use of certain medications. It is more serious in pregnant, old, and very young patients, as well as those with organ dysfunctions. Illegible handwriting and availability of varying dosage forms like slow-release versus normal release also predispose to LASA errors.
The following steps can be taken to prevent LASA errors -
Medication errors: Medication errors are defined as any preventable event that may cause or lead to inappropriate medication use or patient harm while the medication is controlled by the health care professional, patient, or consumer. Medication errors can occur at any stage, including ordering, prescribing, transcribing, dispensing, administering, or monitoring a drug.
Dispensing errors: Dispensing errors refer to medication errors linked to the pharmacy or to whatever health care professional dispenses the medication. Dispensing errors result in deviation from the prescription and can be classified as follows -
| Type | Example |
| Trailing zero | 1.0 mg read as 10 mg |
| Decimal point error | 0.5 mg read as 5 mg |
| Abbreviation errors | AZT (zidovudine) mistaken as azathioprine |
| Sig abbreviations | Misinterpreting “qod” to “qd” or “AD” to “OD” |
The most common reasons for errors include failure to communicate drug orders, illegible handwriting, distractions, wrong drug selection chosen from a drop-down menu, confusion over similarly named drugs, confusion over similar packaging between products, compounding errors or errors involving dosing units or weight. Most prescription errors are caused by illegible handwriting, abnormal doses, early refills, incorrect quantity, incorrect patient, or incorrect drug.
Steps to prevent medication and dispensing errors are as follows:
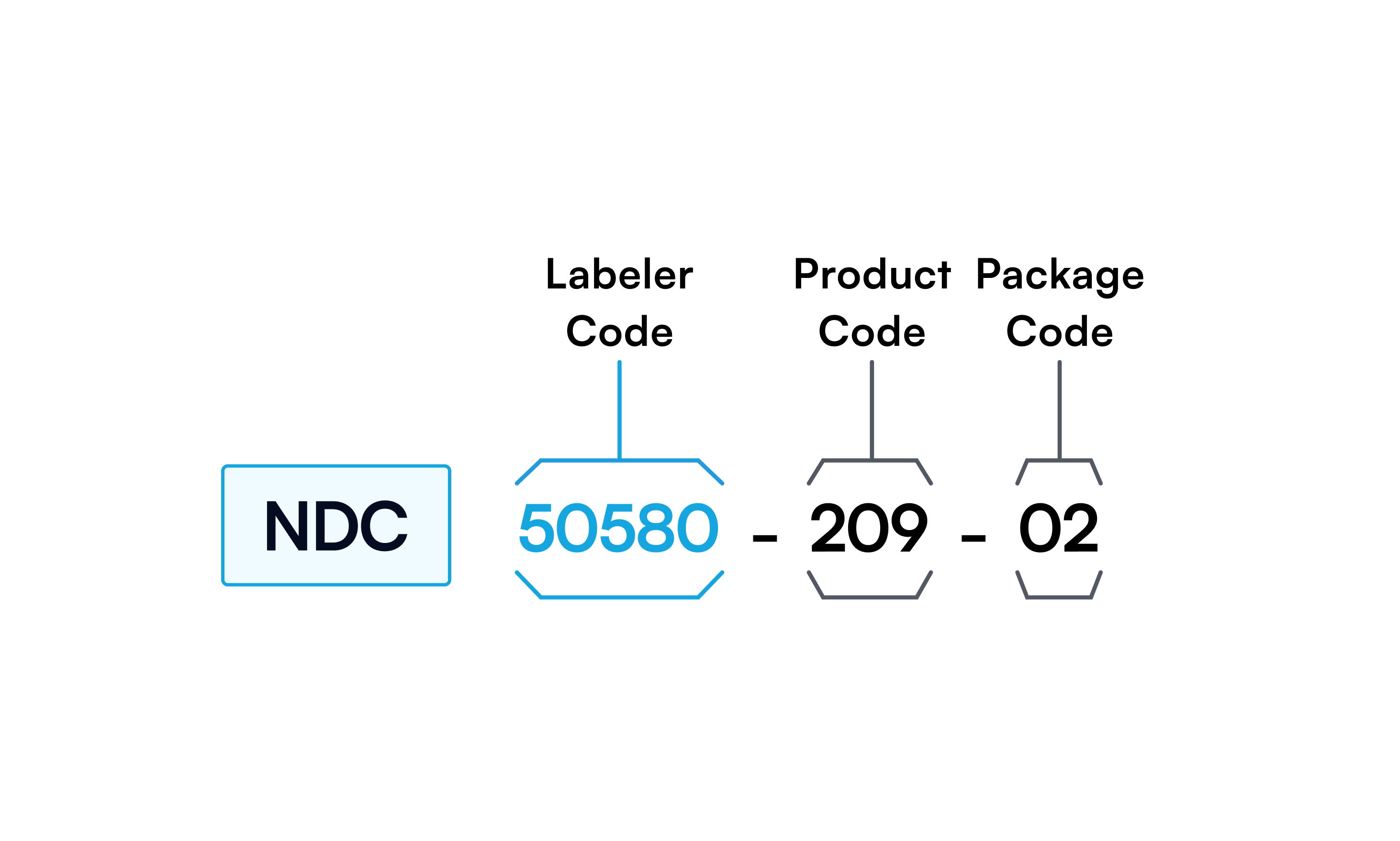
NDC stands for National Drug Code, a unique ten-digit number assigned to prescription and over-the-counter drugs. It helps identify drugs by pharmacy professionals, healthcare providers, and insurance companies. The 10-digit NDC will be in one of the following configurations: 4-4-2, 5-3-2, or 5-4-1. It has three segments: the first segment assigned by the FDA is called a labeler code, the second is called the product code, and the third is the package code. The product code indicates the drug’s strength, form, and formulation. The package code indicates the package form and size. The NDC helps to choose the correct drug and strength; it also identifies drug-drug interactions, reduces errors, and facilitates the processing of drug returns. Generally, the NDC number on the package is a ten-digit number and must be expanded to 11 digits by inserting a “0” in the appropriate spot for insurance and billing purposes. It can be converted into a barcode, making it scannable, known as UPC code. The UPC code is a 12-digit number.
Like adverse events, medication errors must be reported to regulatory agencies, including FDA and state pharmacy boards. The report can be made by healthcare professionals as well as patients. These organizations collectively review error submissions. Case reports are published to educate healthcare professionals regarding errors and near misses. In some cases, the FDA may work with drug manufacturers and others to inform them about concerns with pharmaceutical labeling, packaging, and nomenclature to make appropriate changes to reduce the risk of medication errors.
FDA MedWatch: Patients and healthcare practitioners can use FDA MedWatch to report medication errors and vaccine and drug adverse events. The FDA MedWatch reporting system provides a comprehensive sentry position for many medication errors. All errors need to be reported, whether they caused harm to the patient or not.
FAERS: The FDA Adverse Event Reporting System (FAERS) is a database of adverse events and medication errors reported to the FDA. Healthcare professionals, consumers, and manufacturers can submit reports to FAERS.
VAERS: The vaccine adverse event reporting system, or VAERS, is used to report vaccine-related adverse events. It is co-administered by the FDA and CDC.
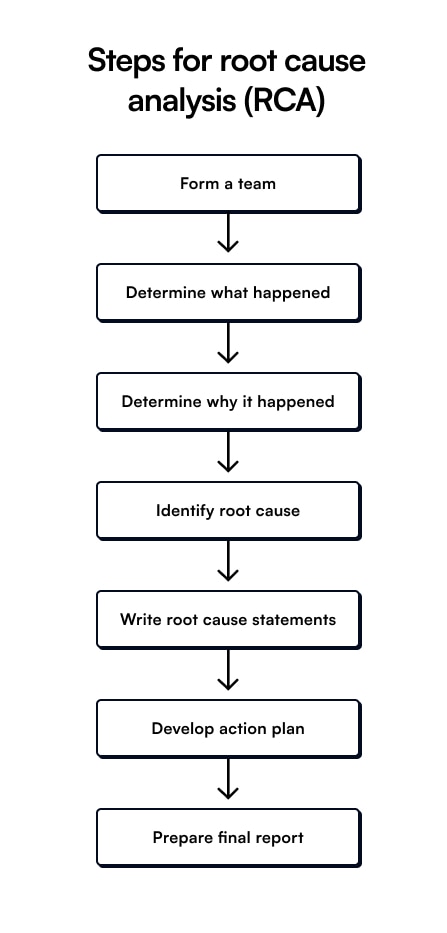
Root-cause analysis (RCA) Root cause analysis consists of the procedures undertaken to find the cause of errors, like medication errors, and suggest preventive measures. It is a regulatory requirement and is essential for all sentinel events. A sentinel event can potentially cause serious harm to the patient and is a result of systematic errors, e.g., look-alike medication leading to incorrect drug dispensing may occur due to a string of mistakes involving prescribing, inefficient storage, quality control, and human error.
Sign up for free to take 10 quiz questions on this topic