1) Silicosis: It results from exposure to crystalline silica when workers chip, cut, drill, or grind objects that contain crystalline silica. High-risk jobs include abrasive blasting, sandblasting, foundry work, stone cutting, rock drilling, quarry work, and tunneling. The following types are seen:
Chronic/classic silicosis: This is the most common form. It occurs after 15-20 years of moderate to low exposure to respirable crystalline silica. Symptoms include shortness of breath with exercise, fatigue, chest pain, or respiratory failure.
Accelerated silicosis: This can occur after 5-10 years of high exposure to respirable crystalline silica. Symptoms include severe shortness of breath, weakness, and weight loss.
Acute silicosis: This occurs after a few months up to 2 years following exposure to extremely high concentrations of respirable crystalline silica. Symptoms include severe, disabling shortness of breath, weakness, and weight loss, and it often leads to death.
In silicosis, the lung is studded with fibrotic nodules. The accompanying pleura is fibrotic and thickened and may show pleural nodules. Microscopically, nodules show central hyalinization, calcification, and cavitation, and they are surrounded by laminated collagen. CxR shows bilateral ground-glass opacities, reticulo-nodular opacities, hilar and mediastinal lymphadenopathy with characteristic “egg-shell calcification,” pleural lesions, and pleural effusion. Changes are more pronounced in the upper lobes. There is an increased risk of tuberculosis. Crystalline silica is a carcinogen and can cause lung cancer. The risk is multiplied in smokers with silicosis.
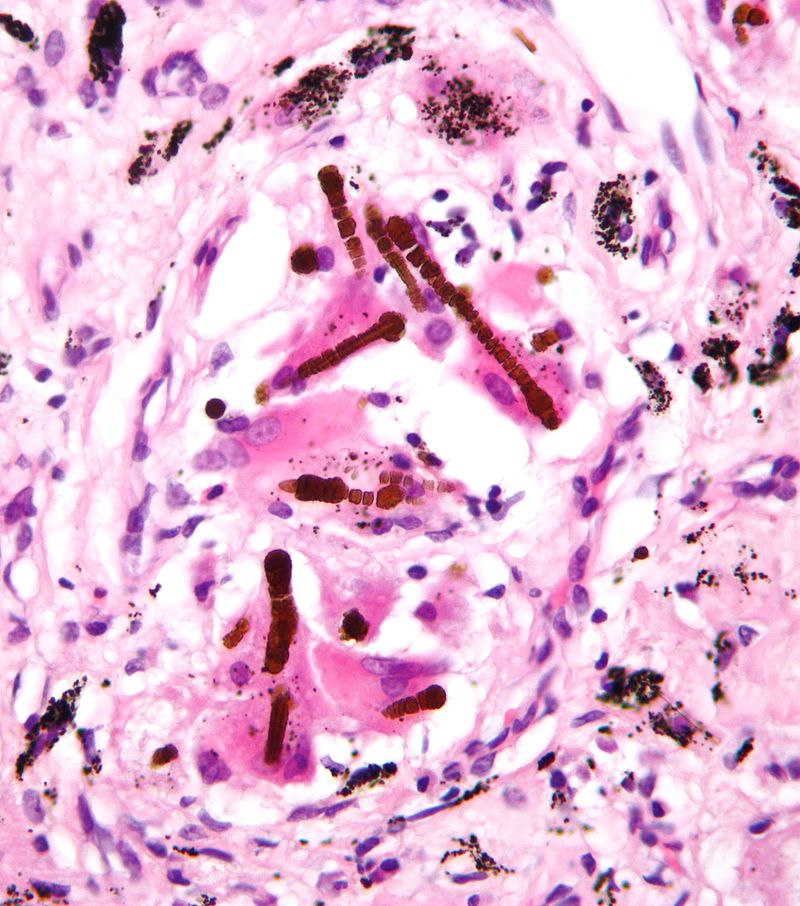
2) Asbestosis: Crocidolite, chrysotile, and amphibole fibres are the most commonly implicated types of asbestos fibres. Asbestosis occurs after occupational exposure to asbestos over a period of years. Asbestos may contaminate clothing and skin and can cause asbestosis in close contacts, such as family members.
As in other pneumoconioses, alveolar macrophages are activated by asbestos fibres. During this process, they release cytokines and growth factors, leading to inflammation and severe fibrosis. Asbestosis is initially asymptomatic and later presents with dyspnea, dry cough, fatigue, clubbing, bibasilar crackles, and cor pulmonale in advanced cases. The disease may progress even after exposure is stopped.
On gross examination, the lungs are small and shrunken and fibrotic, and the pleura is thickened, with prominent changes in the basal areas. Microscopically, asbestos (ferruginous) bodies are seen. Asbestos bodies are dumbbell-, drumstick-, or beaded-shaped, golden brown structures with a transparent, fibrous core made of amphibole-type asbestos fibers coated with glycoproteins and hemosiderin. They stain blue with Prussian blue stain.
Pleural plaques (characteristically on the diaphragmatic pleura) may be present and may be calcified. Pleural fibrosis and effusions may also occur. Asbestosis predisposes to malignant mesothelioma and bronchogenic carcinoma, especially in smokers. Carcinomas of the esophagus, stomach, colon, and kidneys, as well as lymphomas and leukemias, may occur. There is a higher chance of RA (Caplan’s syndrome).
CxR shows bilateral reticular opacities (more pronounced in the lower lobes), pleural plaques, and honeycombing. Nodules and lymphadenopathy are not pronounced. HRCT, lung biopsy, and BAL are preferred investigations for diagnosis.
3) Berylliosis: This is seen after occupational exposure to beryllium via inhalation of airborne beryllium or skin contact in the aerospace, electronics, and nuclear industries. Berylliosis may manifest as beryllium sensitization, chronic beryllium disease (CBD), and lung cancer.
Beryllium sensitization is asymptomatic, but sensitized individuals are at higher risk of developing CBD. CBD is a chronic granulomatous lung disease that presents with dyspnea, unexplained cough, fatigue, weight loss, fever, and night sweats. Symptoms may appear quickly or after a delay of months to years.
Microscopically, noncaseating epithelioid granulomas with Schaumann bodies (similar to sarcoidosis) are seen in the lung. Schaumann bodies are calcium and protein inclusions inside Langhans giant cells in the granuloma. Acute beryllium disease (ABD) is a potentially fatal, rapid-onset form of chemical pneumonia that results from breathing high airborne concentrations of beryllium.
4) Hypersensitivity pneumonitis: Hypersensitivity pneumonitis is an allergic or immune reaction in the lung that occurs in response to a variety of inhaled organic antigens. Many environmental antigens have been implicated, including fungal, bacterial, protozoal, animal, and insect antigens. The most commonly implicated antigens are thermophilic actinomycete species, fungi (Aspergillus and Penicillium), and bird proteins.
It may present in an acute, subacute, or chronic form, depending on the dose of initial antigen exposure. Examples include farmer’s lung, bagassosis, and byssinosis. Polymorphisms in HLA-DR and DQ, TAP genes, etc., determine individual susceptibility to hypersensitivity pneumonitis. Th1 response, IL 12, gamma interferon, IL 17, and type III or IV hypersensitivity contribute to pathogenesis.
Microscopically, infiltration of alveoli with lymphocytes, plasma cells, and macrophages is seen, followed by foreign body granuloma formation. In chronic cases, interstitial fibrosis and honeycombing are seen. Clinical features include cough, dyspnea, fatigue, myalgia, chills, clubbing, low-grade fever, and cor pulmonale. A restrictive pattern is seen on spirometry. CxR may be normal or may show ground-glass opacities; HRCT shows ground-glass opacities, bronchiectasis, or honeycombing.
5) Pulmonary alveolar proteinosis (PAP): This is characterized by buildup of surfactant in the alveoli, which interferes with gas exchange. It may be primary (including autoimmune and hereditary), secondary, or congenital. It presents with dyspnea, peripheral cyanosis, cough, fatigue, malaise, weight loss, and chest pain.
Primary PAP is caused by reduced GM-CSF stimulation of alveolar macrophages, leading to disturbed surfactant homeostasis and surfactant buildup in the alveoli.
In autoimmune PAP (often seen in smokers), B cells produce auto-antibodies to GM-CSF. In hereditary PAP, GM-CSF receptors on alveolar macrophages are defective. It is inherited as an AR disorder.
In secondary PAP, conditions such as myelodysplasia, HIV, chemotherapy, and inhalation injury from silica, titanium, aluminium, etc., damage alveolar macrophages so that surfactant cannot be reabsorbed.
In congenital PAP, mutations are seen in genes coding for surfactant proteins, such as SFTPB, SFTPC, ABCA3, or NKX2.1.
On gross examination, the lungs are filled with turbid fluid. Microscopically, homogeneous, granular, PAS-positive, eosinophilic material with occasional cholesterol clefts fills the alveoli. CxR shows opacities (typically not involving the apical zones and costophrenic angle) in a “batwing” distribution. Some cases may show diffuse consolidation. HRCT shows ground-glass opacities and thickening of interlobular and intralobular septa.
6) Idiopathic pulmonary fibrosis or cryptogenic fibrosing alveolitis: This is a type of diffuse interstitial pneumonia with a poor prognosis, characterized by pulmonary interstitial fibrosis and inflammation not caused by infection or cancer. It is seen more commonly in middle-aged or older males. It presents with dyspnea, chronic dry cough, velcro-like crackles on auscultation, and clubbing. Bilateral reticular infiltrates and hazy opacities are seen on CxR, especially in the lower lung zones.
Mutations in TERT, TERC, PARN, and RTEL1 genes confer higher risk. Overexpression of mucin 5B in small airways is seen. Macrophages containing lamellar bodies are present. Diffuse fibrosis and honeycombing are seen in advanced cases.
7) Hypereosinophilic syndrome (HES): This is characterized by persistent and marked blood eosinophilia for more than 6 months, along with eosinophil-induced organ damage, in the absence of allergic, parasitic, and malignant causes of eosinophilia.
HES can be caused by a sporadic hematopoïetic stem cell mutation leading to primitive clonal expansion of cells in the myeloid lineage with preferential eosinophil differentiation, or by overproduction of eosinophilic poietic cytokines (such as IL5) by an activated population of T cells (Th2 cells).
The skin, heart, lungs, and central and peripheral nervous systems are more commonly involved. Eosinophil cationic protein and major basic protein, peroxidases, elastases, collagenases, and free radicals cause local tissue damage.
Clinical features depend on the organ systems involved and can include urticaria, angioedema, pruritis, heart failure, restrictive cardiomyopathy, endomyocardial fibrosis, peripheral neuropathy, encephalopathy, stroke, pulmonary fibrosis, and hepatosplenomegaly. Therapy is with imatinib, corticosteroids, hydroxycarbamide, alpha interferon, or mepolizumab.
Sign up for free to take 6 quiz questions on this topic